Source: Anton G. Henssen
News • Alveolar rhabdomyosarcoma
Promising new treatment for deadly pediatric tumor
Alveolar rhabdomyosarcoma is a rare pediatric tumor. For more than 40 years there has not been any new development regarding treatment. Research led by Prof. Dr. Anton Henssen at Charité University Berlin has now identified a new therapeutic option, using a drug that is currently under investigation for other types of cancer.
The group observed that alveolar rhabdomyosarcoma cancer cells have high levels of DNA damage and are more dependent to the repair processes than non-cancer cells. This drug blocks the repair of DNA and forces cells to die, thus offering a new option for treating these patients. The project received funding from the Wilhelm Sander Foundation and was recently published.
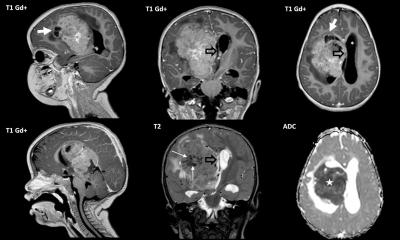
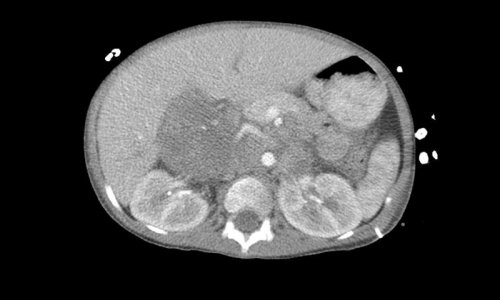
Rhabdomyosarcomas are tumors that grow in the muscle tissue, predominantly in children and adolescents. Even though they are rare, they represent the second most common solid tumor in childhood. Alveolar rhabdomyosarcoma is a type of rhabdomyosarcoma characterized by a chromosome translocation that creates a new protein fusion called PAX3-FOXO1. Alveolar rhabdomyosarcomas are the more aggressive version of rhabdomyosarcoma, with an overall survival rate of 30 percent after five years. In Europe, it is estimated that around 500 new rhabdomyosarcoma cases are diagnosed every year, of which 100 are alveolar rhabdomyosarcoma. Despite many advances in cancer therapeutics, rhabdomyosarcoma treatment has not changed in the last four decades, urging for new and more effective treatments.
One of the challenges in treating alveolar rhabdomyosarcoma is the lack of druggable factors present in the tumor. Besides the PAX3-FOXO1 fusion, alveolar rhabdomyosarcomas do not present many recurrent genetic mutations, thus limiting our ability to use drugs specific for this tumor. Simultaneously, cancer cells frequently present higher DNA damage, which allows the acquisition of new mutations. The damaged DNA needs to be repaired before the cells can divide to ensure that the integrity of the genome is maintained. In recent years, many drugs have been developed to block the repair of the DNA, with promising results in multiple cancer types.
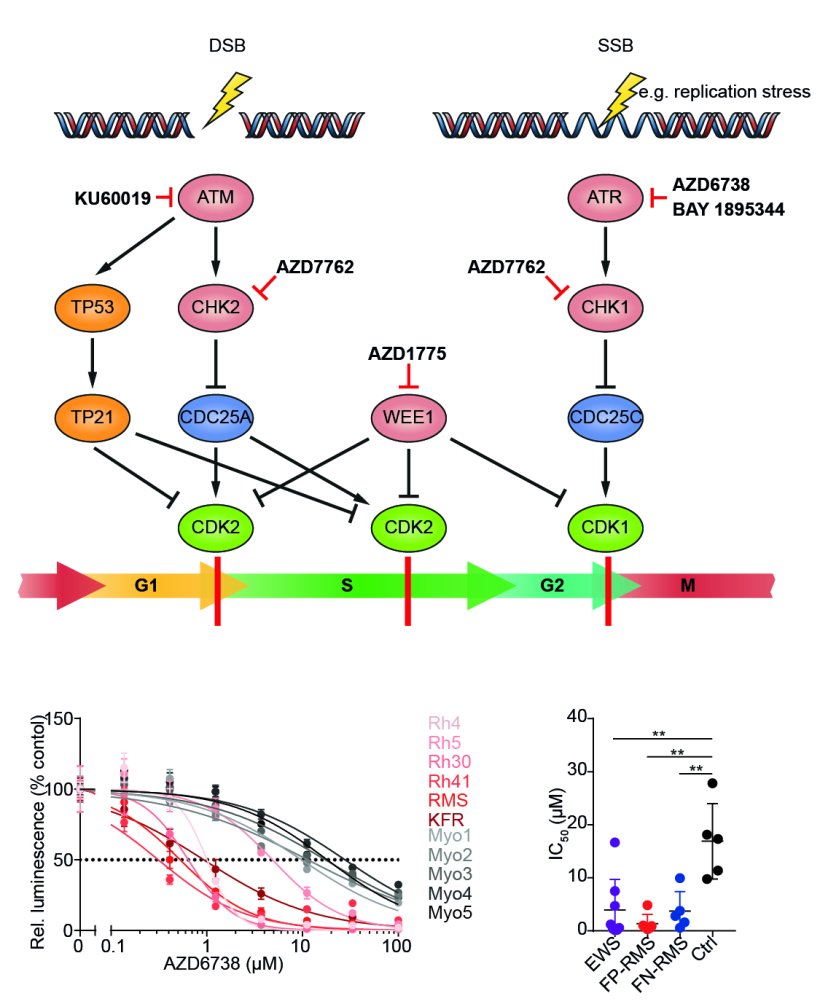
Therapeutic targeting of ATR in alveolar rhabdomyosarcoma
The lab led by Anton Henssen in the Experimental and Clinical Research Center of the Charité University Medicine and Max Delbrück Center, both located in Berlin, has recently shown that alveolar rhabdomyosarcomas are more sensitive to inhibitors targeting ATR, a protein essential for DNA repair. His team identified PAX3-FOXO1 as a factor that increases DNA damage in the cancer cells, therefore making them more vulnerable to DNA damage repair deficiency. By inhibiting ATR, the cells accumulate DNA damage and are forced into cell suicide, so called apoptosis. ”When we tested the drugs in mice that grew alveolar rhabdomyosarcomas derived from a patient we found out that ATR inhibitors had a strong antitumor activity, bringing the drugs one step closer to the patient” says Anton Henssen. Based on this data, a new clinical trial in the US is currently recruiting patients for testing ATR inhibitors in pediatric solid tumors, including alveolar rhabdomyosarcoma.
Given that drug resistance is frequently the limiting factor in cancer therapy, the group started looking into factors that cause resistance to ATR inhibition in alveolar rhabdomyosarcoma. Using a CRISPR screen targeting all genes in the genome, they identified FOSB, a protein that regulates gene expression, as a marker for resistance. The group is now trying to identify new therapies that could overcome this resistance to further improve the treatment of alveolar rhabdomyosarcoma.
These results show that we can exploit what makes these cancer cells more aggressive and turn it into something positive. The potential of ATR inhibitors, alone or in combination with other drugs, including the current treatment of alveolar rhabdomyosarcoma, needs to be further explored in future research, but this study provides a solid starting point that could benefit patients in a few years.
Source: Wilhelm Sander-Stiftung
21.09.2022