News • Congenital defect reconstruction
Pectus Excavatum: First-in-human trial of novel reconstruction scaffold
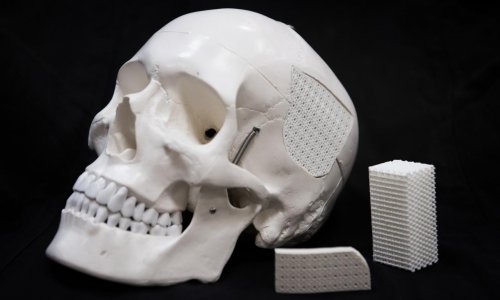
Medtech company BellaSeno announced the initiation of a first-in-human trial of its novel, absorbable soft tissue reconstruction scaffold (Senella).
A patient with Pectus Excavatum congenital defect has undergone surgery at Princess Alexandra Hospital in Brisbane, Australia, earlier this month. The procedure was performed by Dr. Michael Wagels, Principal Investigator of the trial and Plastic and Reconstructive Surgeon at Princess Alexandra Hospital. Primary endpoint of the trial will be the assessment of post-operative adverse device effects (ADE) 12 and 24 months after pectus scaffold implantation. Secondary endpoints cover safety criteria such as adverse events, frequency of complication (short term and long term), number of revision surgeries due to ADEs and pain.
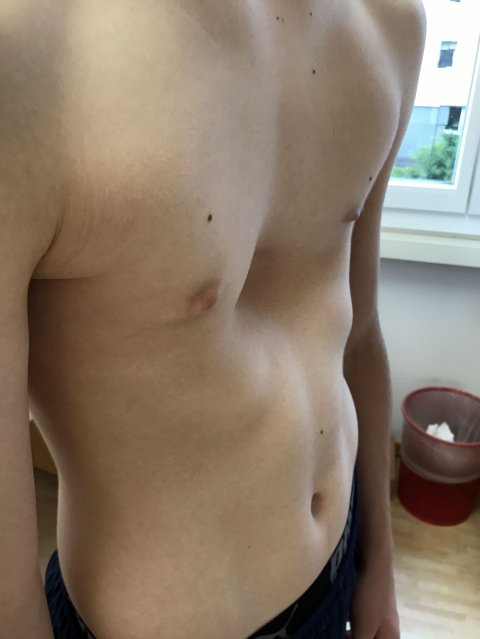
Image source: Gzzz, Pectus excavatum, CC BY-SA 4.0
Pectus Excavatum (or funnel chest syndrome) is a rare malformation of the thorax characterized by a median or lateral depression of the sternum. Funnel chest occurs in 1 to 2% of the population. This is the most common congenital thoracic deformity. Pectus Excavatum is a challenging medical setting and usually requires a two-step surgical process. The condition can impair cardiac and respiratory function and cause pain in the chest and back.
The benefit of using an absorbable scaffold is the simplicity compared to other orthopaedic techniques which use silicone or metal implants. The surgery is designed as a minimally invasive procedure where no bones are touched and the recovery is expected to be less painful than other treatment approaches. Moreover, Senella scaffolds are designed to be fully absorbed by the recipient's body within two years after surgery, leaving no harmful or risky substances.
Moreover, BellaSeno announced that a key patent covering design and porosity features of its Senella platform has been granted in the U.S. The Company has exclusively licensed the patent from Technical University Munich, Germany.

Image source: BellaSeno
“Today marks a major milestone in the history of BellaSeno as we transition into a clinical-stage medtech company,” said Mohit Chhaya, PhD, Chief Executive Officer of BellaSeno. "Our first-in-human study with Senella helps build the foundation for expanding clinical trials to European sites and additional breast reconstruction indications later this year. In addition, we are delighted to further strengthen our global patent portfolio."
"The versatility of our design and manufacturing platform allows us to efficiently produce patient-specific as well as standardized scaffolds," added Navid Khani, PhD, Head of R&D at BellaSeno. "We therefore hope that our approach will transform reconstructive surgery and make a meaningful difference in the lives of patients."
"Our first aim is always the safety and satisfaction of the patient. We are glad that the close collaboration between the surgeons, our supply chain partners and the entire BellaSeno team has made such a rare disease trial possible," said Sara Lucarotti, M.E., Head of Design at BellaSeno.
Source: BellaSeno
21.07.2020