Source: Robert Koch Institute (RKI). Cancer in Germany. Pancreas: 2019-2020; calculation of the quantity of patients has been conducted by the author on basis of the RKI figures.
Sponsored • Brachytherapy in LAPC patients
Innovative Avenue of Treatment – Internal Radiotherapy for Locally Advanced Unresectable Pancreatic Cancer
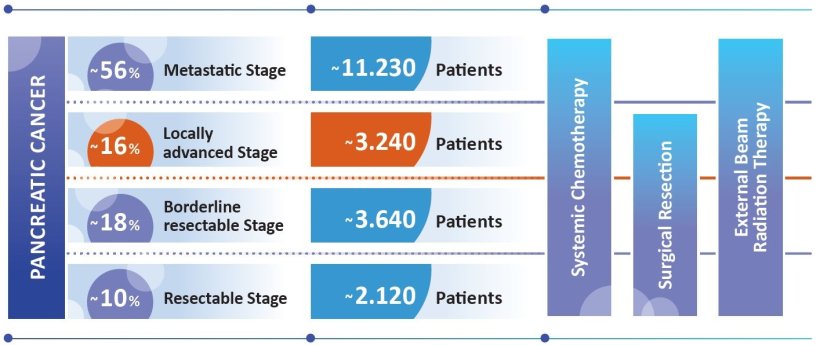
In more than one in six patients diagnosed with pancreatic cancer, the tumour is initially diagnosed at a non-metastatic primarily unresectable, locally advanced stage (LAPC). For these patients, a new internal radiation procedure, OncoSil™ brachytherapy, may become a treatment option – in Germany, around 858 patients could benefit from this innovation annually.¹
OncoSil™ is used in addition to systemic gemcitabine-based chemotherapy that has already been initiated. Compared to external beam radiation therapy, the OncoSil™ treatment is intended to deliver approximately double the radioactivity dose to the pancreatic cancer but without significantly increasing side effects and favouring patient quality of life through a single visit to hospital. The main aim of the treatment procedure is local tumour control, potentially downstage the tumour and ultimately to enable resection of the tumour ultimately.
OncoSil™ has breakthrough therapy status in the EU, the UK and the USA. OncoSil™ is currently being used in Austria, Australia, Belgium, Greece, Israel, Italy, the Netherlands, New Zealand, Spain, Turkey and the UK.
High medical demand
Pancreatic adenocarcinoma is considered the cancer with the most unfavourable prognosis and therapeutic options are extremely limited. Incidence and mortality rates are almost identical. The five-year survival rate is around 10%.1 EU-wide surveys put the figure at only 7%.2 On average, patients fall ill in the middle of their 8th decade of life. The number of cases is rising steadily, due to demographic trends and risk factors such as obesity.3 Pancreatic carcinomas are usually diagnosed at an advanced stage, due to the often non-existent or unspecific symptoms.
The standard of care for patients with inoperable LAPC has long been systemic chemotherapy, with or without radiotherapy. Surgical removal of the tumour is the only potentially curative treatment. However, this is only possible in 15% to 20% of patients.4 Half of all patients have metastases at presentation to healthcare providers, which rules out surgical resection. These patients have a median life expectancy of only around 7 to 11 months.5 For the vast majority of patients, it is a matter of controlling the disease and maintaining quality of life, without the possibility of a cure.6,7,8
Previous study results
OncoSil™ has presented positive results on tolerability, safety and efficacy in six clinical trials. The studies also show that adding OncoSil™ causes a negligible additional safety burden on top of the safety profile of chemotherapy alone.9
The PanCO study9 showed that physicians can achieve local disease control in more than 90% of patients with unresectable LAPC over 16 weeks and median survival to 15.2 months with OncoSil™ in addition to chemotherapy.9
In addition, OncoSil™ (plus chemotherapy) was able to convert previously inoperable tumours into potentially resectable disease in one-in-three patients (33.3%), and enabled successful surgical resections in almost one-in-four patients (23.8%) related to per-protocol population (20% related to intention-to-treat population) with R0 resection margins in 8 out of 10 cases.2 These are clinically relevant results, as surgical removal of the cancerous tissue can improve the three-year survival rate of an originally inoperable LAPC from 11% to 50%.10
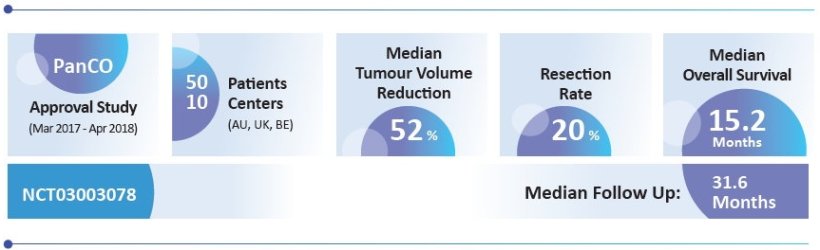
All findings presented relate to the intention-to-treat population. With relation to per-protocol population the figures are: resection rate 23,8%, median overall survival 15,5 months.
Handling the OncoSil™ implant
The OncoSil™ treatment involves a team of an oncologist, an abdominal surgeon, an endoscopist and a nuclear medicine physician. The specialist physicians performing the procedure will have previously been instructed in accordance with a training programme provided by OncoSil Medical.
There is a great unmet medical need for treatment of locally advanced pancreatic cancer. In this regard, it is important to investigate new avenues of treatment. We certainly hope that brachytherapy may prove to be effective at this stage of disease
Volker Heinemann
OncoSil™ consists of a large number of tiny microparticles containing the pure beta emitter radio-isotope phosphorous-32. The radiating microparticles are implanted directly into the target tumour via injection under endoscopic ultrasound guidance and is designed to provide an absorbed dose of 100 Gy of radiation to the tumour. The device emits 98% of the radiation within 81 days.11 The positioning of the microparticles is confirmed using a SPECT-CT Bremsstrahlung scan.9,11
Prof. Dr. med. Volker Heinemann, Director of the Cancer Center – CCC MunichLMU – Comprehensive Cancer Center: "There is a great unmet medical need for treatment of locally advanced pancreatic cancer. In this regard, it is important to investigate new avenues of treatment. We certainly hope that brachytherapy may prove to be effective at this stage of disease."
Availability of OncoSil™ therapy
The OncoSil™ therapy might become available in Germany as part of a coverage with evidence development (CED) randomised study soon. The federal joint committee (G-BA) is currently discussing the development of the corresponding study design.12 This CED study would likely feature a comparison of the guideline recommended systemic chemotherapy regimens FOLFIRINOX or gemcitabine plus nanoparticle albumin bound (nab)-paclitaxel versus the addition of OncoSil™ to the systemic chemotherapy regimens.
In parallel, there is growing interest of German hospitals to offer the OncoSil™ treatment for inpatient hospitalisation. In 2022, 25 hospitals were applying for NUB funding, which grew to 36 hospitals in 2023 and in 2024, 84 hospitals finally were granted this entitlement to try to negotiate a supplementary fee to fund the innovative OncoSil™ treatment option with the statutory health insurance companies during the annual hospital budget negotiations.
About OncoSil™
OncoSil Medical is a medical technology company that aims to improve the treatment of patients with pancreatic cancer. The OncoSil™ device consists of microparticles containing Phosphorous-32 (32P) which are delivered by injection directly into a patient’s unresectable locally advanced pancreatic cancer tumour under endoscopic ultrasound guidance and in addition to gemcitabine-based chemotherapy.
OncoSil™ was approved on March 30, 2020 as an active, implantable medical device in accordance with Directive 90/385/EEC with CE certificates 718878 and 718879 for the European Economic Area.
References:
- Robert Koch Institute (RKI). Cancer in Germany. Pancreas: 2019-2020. Retrieved 22.04.2024
- Cancer Research UK. Pancreatic cancer. Survival. Retrieved September 2020.
- Robert Koch Institute. Cancer in Germany. Pancreas: 2019-2020.
- Ducreux M et al. Ann Oncol 2015; 26 (Suppl 5): v56-68.]
- Robert Koch Institute. Cancer in Germany. Pancreas: 2017-2018.
- Ducreux M et al. Ann Oncol 2015; 26 (Suppl 5): v56-68.
- Balaban EP et al. J Clin Oncol 2016; 34: 2654-2668.
- Cancer Australia. Optimal care pathway for people with pancreatic cancer. 2016. Accessed July 2020.
- Ross PJ, Wasan HS, Croagh D, Nikfarjam M, Nguyen N, Aghmesheh M, et al. Results of a single-arm pilot study of 32P microparticles in unresectable locally advanced pancreatic adenocarcinoma with gemcitabine/nab-paclitaxel or FOLFIRINOX chemotherapy. ESMO Open 2022; 7 (1): 100365.
- Gemenetzis G et al. Ann Surg 2019; 270: 340-347.
- OncoSil™ System – Instructions for Use
- Trial guideline on ultrasound-guided, endoscopic intratumoral injection-implantation of ³²P-labeled microparticles in irresectable, locally advanced pancreatic tumors combined with neoadjuvant gemcitabine- or folfirinox-based chemotherapy
Source: OncoSil Medical
04.06.2024