Molecular imaging
Report: David Loshak
Molecular imaging, the discipline that unites molecular biology and in vivo imaging technologies to assess biological activity in the body, promises to open up ‘…an entire new universe,’ declared Dr Ralph Weissleder, of the Centre for Molecular Imaging Research at Massachusetts General Hospital, USA, in the journal Radiology. That was just one decade ago. And he was right. It has indeed done that.
Imaging technologies today are applied in many ways to a score of biomedical applications, says Dr Mark Lythgoe, director of the Centre for Advanced Biomedical Imaging at University College, London, UK. ‘We seek to use novel in vivo imaging technologies to further understand the mechanisms of disease and develop therapeutic strategies. Furthermore, we aim to deliver a multimodal imaging programme to investigate the molecular, functional and structural consequences of the disease process on a range of different scales.’
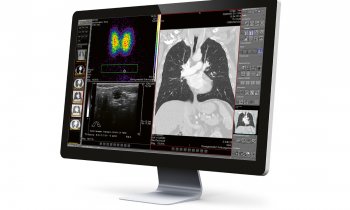
The Centre provides high-field magnetic resonance imaging (MRI), a photo-acoustic imaging facility, in vivo 2-photon laser-scanning microscopy, ultrasound, bioluminescence and fluorescence imaging, computerised tomography (CT) combined with single photon emission computed tomography (SPECT) and positron emission tomography (PT), all enabling cross-disciplinary work in neuroscience, cardiovascular biology and oncology.
‘The development of imaging technology is an essential part of the translational pipeline for drug development and personalised medicine, and is important to research in the pharmaceutical industry,’ Dr Lythgoe adds. ‘Such strategies will help to remove major bottlenecks in applying new discoveries to the clinic. They will generate the knowledge and understanding to transform human health and well-being.’
As Dr Weissleder predicted, molecular imaging enables earlier detection and characterisation of disease. This means that relatively gross parameters, such as tumour burden and anatomic location, can be improved with specific parameters, such as detection of premalignant molecular abnormalities, angiogenesis growth factors, tumour cell markers or genetic alterations.
Such imaging assessment allows, with new targeted therapies, assessment of therapeutic effectiveness at a molecular level well before phenotypic changes occurred - the study of pathogenesis in intact micro-environments of living systems. And it provides three-dimensional information far faster than had been possible with time-consuming, labour-intensive, invasive conventional techniques such as histological analysis.
In only a decade or so then, molecular imaging has revolutionised the practice of medicine and patient care. It provides earlier than ever detection by disclosing information that would otherwise require exploratory surgery or costly diagnostic tests (if available). And, by helping to understand the basis of disease, not just the end result, it even promises to help prevent disease.
Molecular imaging today encompasses multiple image-capture techniques, cell/molecular biology, chemistry, pharmacology, medical physics, biomathematics and bio-informatics, observes Professor Sanjiv Sam Gambhir, head of nuclear medicine at Stanford University, California, USA. Nuclear medicine he explains, ‘…uses radio-labelled molecules (tracers) that produce signals by means of radioactive decay only. But it also uses those and other molecules to image via means of sound (ultrasound), magnetism (magnetic resonance imaging) or light (bioluminescence and fluorescence), as well as other emerging techniques. Nuclear imaging has established itself as an indispensable tool in pre-operative diagnostics’.
Several other fields offer a range of imaging technologies to produce signals. These vary in five key respects - spatial resolution, depth penetration, energy expended for image generation (ionising or non-ionising), availability of injectable/biocompatible molecular probes, and the detection threshold of probes for a given technology.
Because each imaging technique has its advantages and drawbacks, Prof. Gambhir notes, a variety of approaches are needed for the increasingly sophisticated biological interrogation of cells that molecular imaging now offers.
For example, PET, which uses high energy gamma rays for image generation, has high sensitivity but low spatial resolution. By contrast, SPECT uses low energy gamma rays but, also, has low spatial resolution to set against its capacity to image multiple probes simultaneously.
Optical bioluminescence and optical fluorescence imaging use visible light or near-infrared; both have high sensitivity but low spatial resolution. On the other hand, MRI uses radio waves to generate morphological and functional imaging but has low sensitivity and requires long scan and post-processing time.
CT employs X-rays for anatomic imaging (bone and tumour) but has limited molecular applications and limited soft tissue resolution. Ultrasound has the advantages of real time imaging and being cheap. It has limited spatial resolution but can be used in photo-acoustic imaging to disclose tissue on an mm-cm length scale.
The proven value and even greater potential of imaging in intra-operative procedures is exemplified by a new suite at the Health Sciences Centre, Winnipeg, Canada, which provides multi-modality image guidance capabilities. It includes an interventional theatre for neurovascular procedures such as stroke management, an operating room for neurosurgery and a diagnostic centre.
Developed by the Canadian company IMRIS, a leading provider of image guided therapy, its systems feature fully integrated surgical and interventional suites that incorporate MRI, CT and fluoroscopy for intra-operative imaging during neurosurgical, cardiovascular and neurovascular procedures.
The suite includes a bi-plane angiography system and an MR scanner that can be moved readily from imaging to surgery or intervention without transporting the patient, ensuring the optimum position for all procedures. MR images can be taken before and during procedures to assess tissue condition and can also be used with fluoroscopic images during neurovascular procedures.
Another significant recent development in this field has been the facility to use high-resolution imaging and guidance via optical coherence tomography. That permits transfer of diagnostic capabilities from the pathology lab to the operating theatre, enabling real-time tissue visualisation and point-of-care decisions.
21.05.2010