Source: Science China Press
News • Regenerative medicine
Injectable stem cell assembly for cartilage regeneration
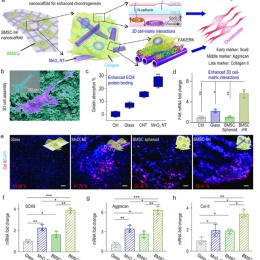
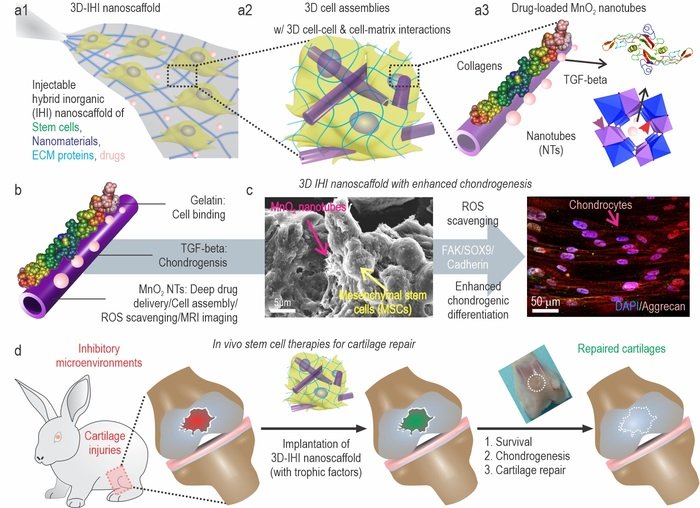
Researchers led by Prof. Qiuyu Zhang (Northwestern Polytechnical University), Prof. Ki-Bum Lee (Rutgers University), and Prof. Liang Kong (School of Stomatology, The Fourth Military Medical University) have established an injectable hybrid inorganic (IHI) nanoscaffold-templated stem cell assembly and applied it to the regeneration of critically-sized cartilage defects.
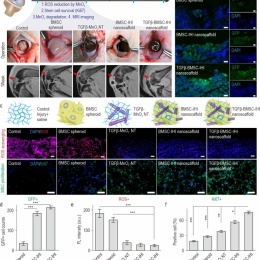
Cartilage injuries are often devastating and most of them have no cures due to the intrinsically low regeneration capacity of cartilage tissues. The rise of 3D stem cell culture systems has led to breakthroughs in developmental biology, disease modeling, and regenerative medicine. For example, stem cells, once transplanted successfully, could initially secret trophic factors for reducing inflammation at sites of cartilage injuries and then differentiate into cartilage cells (e.g., chondrocytes) for functional restoration. Nevertheless, there are critical barriers remaining to be overcome before the therapeutic potential of stem cell therapies can be realized. The limited control over the chondrogenic differentiation of stem cells in vivo has often resulted in compromised regenerative outcomes. Moreover, due to the prevalence of oxidative stress and inflammation in the microenvironment of injury sites, stem cells frequently undergo apoptosis after injection.
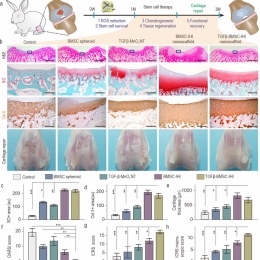
To address these challenges, they demonstrated the development of a 3D IHI nanoscaffold-templated stem cell assembly system for advanced 3D stem cell culture and implantation. 3D-IHI nanoscaffold rapidly assembles stem cells into injectable tissue constructs through tailored 3D cell-cell and cell-matrix interactions, deeply and homogeneously delivers chondrogenic proteins in the assembled 3D culture systems, and controllably induces chondrogenesis through nanotopographical effects. Once implanted in vivo in a rabbit cartilage injury model, 3D-IHI nanoscaffold effectively modulates dynamic microenvironment after cartilage injury through the integration of the aforementioned regenerative cues, and simultaneously scavenges reactive oxygen species using a manganese dioxide-based composition. In this way, accelerated repair of cartilage defects with rapid tissue reconstruction and functional recovery is realized both in the short term and long term. Given the excellent versatility and therapeutic outcome of 3D-IHI nanoscaffold-based cartilage regeneration, it may provide promising means to advance a variety of tissue engineering applications.
Source: Science China Press
20.04.2022