© HHU / Selene Lickfett
News • Neurometabolism
Huntington: New insights into disease progression
Researchers from the Max Delbrück Center in Berlin and Heinrich Heine University Düsseldorf (HHU) have implicated a new gene in the progression of Huntington’s disease in brain organoids.
The gene may contribute to brain abnormalities much earlier than previously thought, as they now report in “Nature Communications”.
For the first time, researchers have implicated the gene CHCHD2 in Huntington’s disease – an incurable genetic neurodegenerative disorder – and identified the gene as a potential therapeutic target. In a brain organoid model of the disease, they found that mutations in the Huntington gene HTT also affect CHCHD2, which plays a role in maintaining the normal function of mitochondria.
Six different labs at the Max Delbrück Center under the lead of Dr Jakob Metzger from the “Quantitative Stem Cell Biology Lab” and the “Stem Cell Metabolism” research group of Professor Dr Alessandro Prigione from the Medical Faculty at HHU took part in the study. Each group contributed its unique expertise on Huntington’s disease, brain organoids, stem cell research and genome editing.
“We were surprised to find that Huntington’s disease can impair early brain development through defects associated with mitochondrial dysfunction,” says Dr Pawel Lisowski, one of the lead authors from the lab of Dr Metzger at the Max Delbrück Center. “The organoid model suggests that HTT mutations damage brain development even before clinical symptoms appear, so it is very important to detect this late-onset neurodegenerative disease early,” continues Selene Lickfett, a further lead author of the study and doctoral student from the research group of Professor Prigione at HHU.
Recommended article
Article • Cerebral insights
The brain: mysterious grey matter
More than 80 billion neurons, trillions of synapses and almost 6 kilometres of neural pathways: The brain is an anatomical masterpiece – and still puzzles science. Keep reading to find out about latest research and therapies of brain diseases.
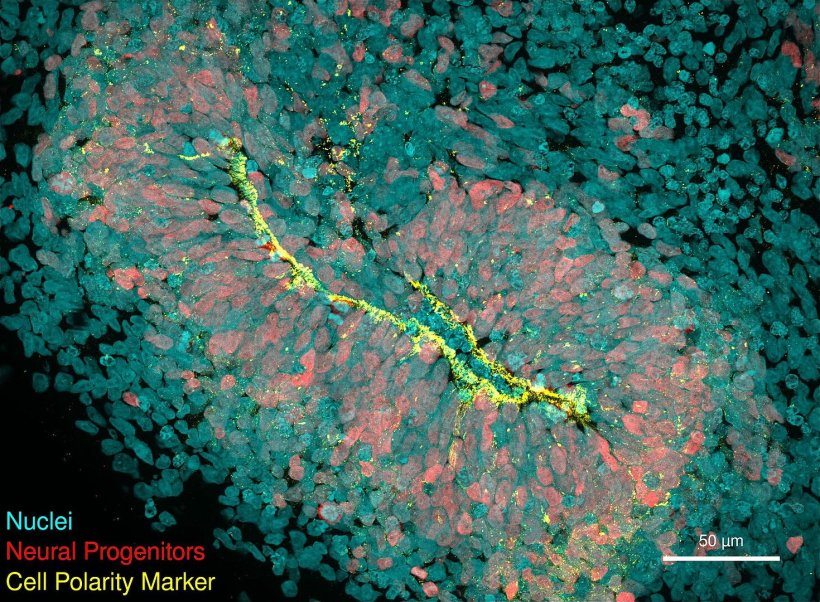
An organoid is a three-dimensional, organ-like structure, which researchers grow from stem cells in the laboratory. Depending on the disease and research question, organoids can be cultivated from different types of tissue. Measuring just a few millimetres in size, they mirror the interaction of multiple cell types. No another model grown in a petri dish can supply such complex data on cell functions in the human body.
In Huntington’s disease, the Huntington gene HTT exhibits an unusually high number of CAG repeats – the three letters for the nucleotides cytosine, adenine and guanine. People with 35 or fewer repeats are generally not at risk of developing the disease, while carrying 36 or more repeats has been associated with the disease. “The greater the number of repeats, the earlier the disease symptoms are likely to appear,” explains Metzger, senior author of the study. The mutations cause nerve cells in the brain to die off on a progressive basis. Over time, those affected steadily lose muscle control and develop psychiatric symptoms such as impulsiveness, delusions and hallucinations. Huntington’s disease affects approximately five to ten in every 100,000 people worldwide. Currently available therapies only treat the symptoms of the disease; they do not slow its progression or cure it.
The prevalent view is that the disease progresses as a degeneration of mature neurons. But if changes in the brain already develop early in life, then therapeutic strategies may have to focus on much earlier points in time
Alessandro Prigione
To study how mutations in the HTT gene affect early brain development, Lisowski first used variants of the CRISPR-Cas9 gene editing technology and manipulated the DNA repair pathways to modify healthy induced pluripotent stem cells in such a way that they carried a large number of CAG repeats. This process was technically challenging as gene editing tools are not efficient in gene regions that contain sequence repeats such as the CAG repeats in HTT, says Lisowski.
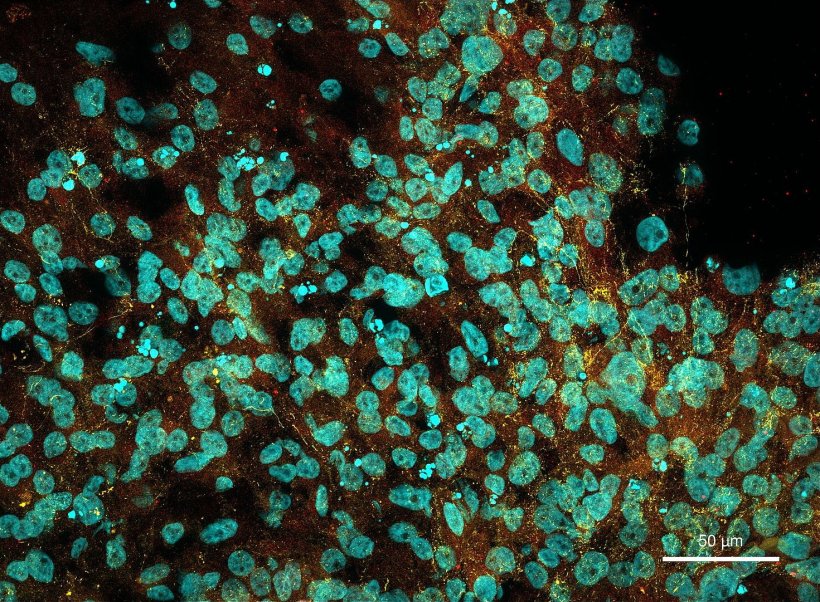
The researchers then grew brain organoids – three-dimensional structures that resemble early-stage human brains – from the genetically modified stem cells. When they analysed gene expression profiles of the organoids at different stages of development, they noticed that the CHCHD2 gene was consistently underexpressed, which reduced the metabolism of the neuronal cells. In healthy people, CHCHD2 ensures the health of the mitochondria – the energy producing structures in cells. CHCHD2 has been implicated in Parkinson’s disease, but never before in Huntington’s.
The researchers were able to reverse the harmful effect on the neuronal cells when they restored the function of the CHCHD2 gene. “That was surprising,” says Selene Lickfett. “It suggests in principle that this gene could be a target for future therapies.”
© HHU / Selene Lickfett
“In addition, defects occurred in neural progenitor cells and brain organoids before potentially toxic aggregates of the mutated Huntingtin protein had developed,” adds Metzger, indicating that the disease pathology in the brain begins long before it becomes clinically evident. The prevalent view is that the disease progresses as a degeneration of mature neurons,” says Prigione. “But if changes in the brain already develop early in life, then therapeutic strategies may have to focus on much earlier points in time.”
“Our genome editing strategies, in particular those that eliminate CAG repeats in the Huntington gene, are highly promising and succeeded in reversing some of these developmental defects. This paves the way for a potential gene therapy approach,” says Prigione. “Another potential approach for therapies could be to increase CHCHD2 gene expression,” he adds. “The findings may also have broader applications for other neurodegenerative diseases. Early treatments that reverse the mitochondrial phenotypes shown here could be a promising avenue for counteracting age-related conditions like Huntington’s disease.”
Source: Heinrich Heine University Düsseldorf, text: Arne Claussen
24.08.2024