Image source: VIB; image by Dr. Tim Vangansewinkel
News • Disturbed lipid metabolism
Charcot-Marie-Tooth: Researchers uncover impact of genetic cause
Charcot-Marie-Tooth disease (CMT), a group of heritable disorders that affect the peripheral nerves, is marked by specific genetic changes.
Research by the team of Prof. Ludo Van Den Bosch (VIB-KU Leuven) now reveals the effects of one such genetic cause. They found that the duplication of the gene PMP22 causes problems in the cell membrane of Schwann cells that provide the insulating cover for nerves. The results appeared in the journal Brain.

Image source: VIB
Charcot-Marie-Tooth disease is a group of inherited disorders that affect the peripheral nerves, leading to muscle weakness and sensory loss in the extremities. Among the various subtypes of CMT, CMT1A is the most common form, characterized by a duplication of the PMP22 gene. Despite being a well-known genetic abnormality associated with CMT1A, the precise mechanisms by which PMP22 duplication contributes to the disease have remained elusive until now.
The PMP22 gene codes for ‘peripheral myelin protein 22’, a protein that is part of the myelin sheath – the protective cover – of peripheral nerves. That myelin sheath degrades in CMT1A. Because the PMP22 protein is produced by Schwann cells, that's where the lab of Prof. Ludo Van Den Bosch (VIB-KU Leuven Center for Brain & Disease Research) focused their attention. By investigating human cell cultures and animal models of CMT with the PMP22 duplication, the researchers could assess the impact of PMP22 duplication on developing Schwann cells.
Starting from induced pluripotent stem cells (iPSCs) differentiated into human Schwann cells and using advanced imaging techniques and molecular analyses, the researchers were able to elucidate the intricate pathways through which PMP22 duplication dysregulates lipid metabolism and disrupts the normal functioning of Schwann cells.

Image source: VIB
Dr. Robert Prior, co-first author of the study (previously VIB-KU Leuven, now UKB Bonn, Germany), said: “One of our key findings was the identification of dysregulated lipids in the plasma membrane of developing human Schwann cells carrying the PMP22 duplication. This impairs the structural integrity and bending properties of the plasma membrane, compromising the ability of the Schwann cells to ‘wrap’ around the peripheral nerves, producing a lipid-rich cover called myelin. This myelin sheath electrically insulates the nerves and Schwann cells also provide metabolic support. The dysregulation of lipids in the plasma membrane finally allows us to understand why the communication between CMT1A patient Schwann cells and peripheral nerves breaks down, even before the onset of myelination.”
Moreover, the researchers discovered that targeting the dysregulated lipid pathways in Schwann cells could potentially reverse some of the detrimental effects of the PMP22 duplication. By modulating lipid metabolism and restoring plasma membrane organization, novel therapeutic strategies could be developed to alleviate the symptoms of CMT1A and to improve the quality of life for affected individuals.
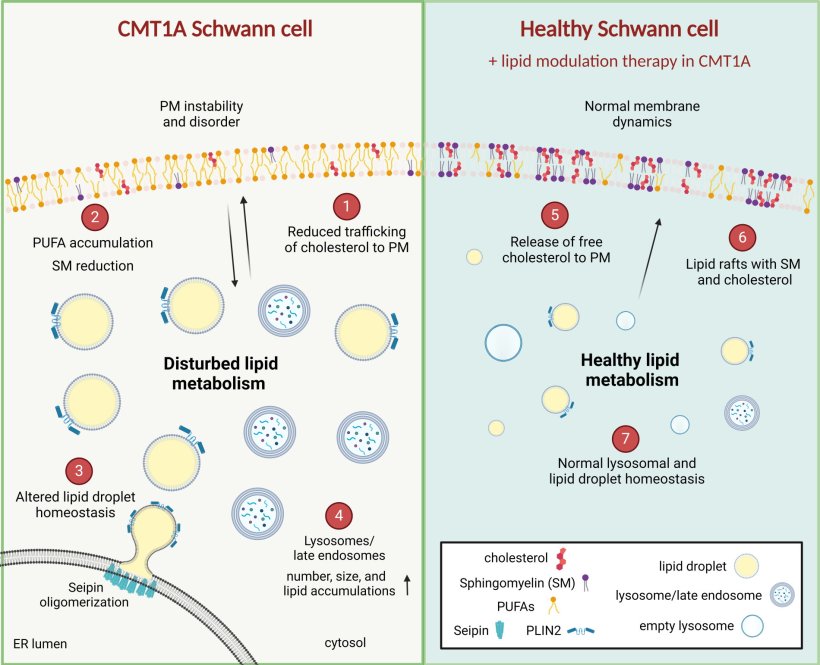
Image credit: Alessio Silva, Robert Prior, Tim Vangansewinkel
Prof. Van Den Bosch added: "Using patient-derived cells, our work provides a foundation for the development of targeted therapies that address the underlying molecular defects in CMT1A. By understanding how PMP22 duplication disrupts lipid homeostasis, we can now explore innovative approaches to restore cellular function and potentially halt the progression of this devastating disease."
As researchers continue to explore the complexities of genetic disorders like CMT1A, new opportunities emerge for the development of therapies that target the root causes of disease. In this case, the effect of a gene duplication on developing Schwann cells points the way ahead to future therapeutic interventions.
Source: Vlaams Instituut voor Biotechnologie
14.06.2024