Healing skin wounds
Researchers define the role of signal molecule c-Met

c-Met – the signal molecule that regulates cell growth and cell migration during embryonic development – has been shown to play a key role in healing in the skin, according to a paper published in the Journal of Cell Biology ( Vol.177, Nr. 1, pp. 151 – 162, 2007) by PhD student Jolanta Chmielowiec, working with Professors Walter and Carmen Birchmeier at the Max Delbrück Centre for Molecular Medicine (MDC) in Berlin, Germany. The research demonstrated that if
c-Met is missing in skin cells, no new tissue can form to close a wound.
When the skin is injured, it first scabs over, sealing the wound. Starting from the edge of the wound, keratinocytes then migrate across the wound, proliferating very quickly to rapidly form new skin tissue – hyperproliferative epithelium – which also fills the wound with new skin cells so that new tissue can replace the scab.
c-Met regulates this migration process from the edge of the wound. It is a receptor molecule also localized on epithelial cell membranes.
Professor Carmen Birchmeier and her research team have studied the role c-Met plays in developmental biology for several years. Interacting with c-Met is a growth factor named hepatocyte growth factor/scatter factor (HGF/SF) because it was found to be a growth factor in hepatocytes (liver cells). The liver regenerates particularly quickly after injury. In cancer research, this factor also plays a key role as scatter factor, which Professor Walter Birchmeier and his colleagues demonstrated repeatedly.
The duo HGF/SF and c-Met is crucial in regulating cell migration. Together, the two are not only released in the liver, but also in the lung, the kidneys, and the heart when these organs are injured. As MDC researchers were able to show, this is also the case with skin wounds: HGF/SF and c-Met are increasingly released by the hyperproliferative skin tissue. Hence, this tissue promotes its own growth. However, while c-Met is normally found in both the skin and the hair follicles (and in wounds is increasingly released in the hyperproliferative epithelium), HGF/SF is proven to be present prior to injury in the hair follicles but not in the skin. HGF/SF is not active in the skin until after an injury at which time it is particularly active at the wound edges of the hyperproliferative epithelium.
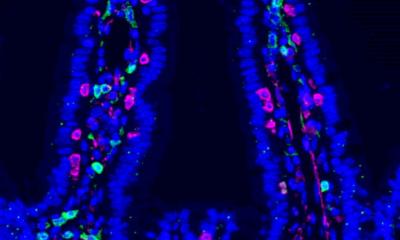
With a special technique, the MDC researchers specifically de-activated the gene for c-Met in mice. They discovered that mice whose skin cells no longer produce c-Met do not form new skin when the skin is injured. In mice that still have some skin cells with active c-Met, because those cells escaped the genetic mutation, wound healing is not blocked. However, it starts later and takes twice as long as usual. This means that only skin cells with active c-Met can build up fast-growing, protective new tissue to close a skin wound.
Corresponding author:
Walter Birchmeier.
E-mail: wbirch@mdc-berlin.de
31.08.2007