COMPAMED 2011 - Trend Report
Modern medical technology is evidently held in high esteem by the general population. In a recent survey conducted by the market research institute Emnid commissioned by the industry association SPECTARIS, about 80% of the patients surveyed said that under certain circumstances they would be willing to pay more for their health insurance in return for consistent treatment with state-of-the-art and innovative medical technology. In spite of the existing cost pressures, in particular in the primary (public) health care market, it is thus safe to assume that there is still a high level of interest in advanced diagnostic procedures and therapies amongst medical users and their patients.
Suppliers play an important role in this field in terms of the development of novel medical devices, systems and methods, as is demonstrated impressively each year by the trade fair COMPAMED – High tech solutions for medical technology in Düsseldorf.
As the leading market platform for suppliers, covering a range of topics reaching all the way from new materials and components to systems and production outsourcing and services, it is still growing and will take place from 16 - 18 November in halls 8a and 8b of the Düsseldorf Exhibition Centre in parallel to the world's largest medical trade fair MEDICA (16 - 19 November 2011).
“On the basis of the excellent response and registrations to date, at least 600 exhibitors are expected to book about 10,000 square metres of space”, says Joachim Schäfer, Managing Director of Messe Düsseldorf GmbH, explaining the slight increase in the number or registrations relative to 2010. The drivers for this positive development, which reflects the upward trend in the medical technology industry, include the rapid growth in the world's population and demographic change, which is leading to rapidly ageing societies, at least in the industrialised nations, but is also resulting in a general increase in awareness of medical issues and increasing prosperity in emerging countries.
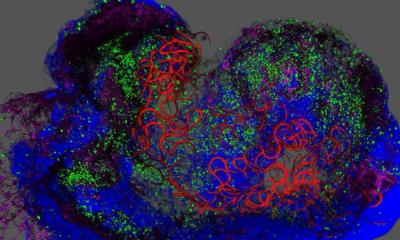
Regenerative medicine, for example, is gaining ever-increasing importance, in particular in the light of demographic change. One of the aims of regenerative medicine is to grow artificial skin for transplantations or for testing cosmetics and chemicals. At present it is being produced manually on a laboratory scale, with cultivation taking six weeks. So far, even well-established international corporations have not yet managed to make more than 2,000 pieces of skin, each one just one square centimetre in size, per month. Four Fraunhofer institutes have now developed the first fully automated sterile system for producing skin faster and in larger quantities. “We have succeeded in creating the world’s first ever totally integrated process chain in a single system– from the cell extraction and cell proliferation through to three-dimensional tissue formation”, explains Professor Heike Walles, Head of the Department of Cell and Tissue Engineering at the Fraunhofer Institute for Interfacial Engineering and Biotechnology (IGB) in Stuttgart. The skin factory aims to produce 5,000 skin equivalents the size of postage stamps per month. “Our great advantage is that we are able to produce both a dermal and an epidermal part of the skin, in other words both the inner as well as the outer layer of skin”, she continues. But that is just the beginning. They aim to develop the technology, which is based on stem cells, further in the years ahead so that they will also be able to use it to produce other kinds of tissue such as cartilage automatically. In the long term, the researchers even hope to make it possible to produce entire organs in the lab.
Supporting scaffolding for groups of cells using special lasers
The Fraunhofer Institute for Laser Technology (ILT) in Aachen is pursuing one approach towards producing tissue implants. Using a special laser technique, researchers at the ILT, which will be exhibiting at COMPAMED 2011 (Hall 8a, booth F 19), and other Fraunhofer Institutes have succeeded in producing hybrid biomimetic structures, which can be used as a basis for scaffold and implant structures. The structures emulate the body’s own tissue as closely as possible. This has enabled biologists to meet one of the key requirements for generating tissue implants, which will provide ideal conditions for the cells to colonize and grow, in the future. To achieve this, the researchers have transferred the rapid prototyping technique to endogenous materials. They combine organic substances with polymers and produce three-dimensional structures. As the basis they use dissolved proteins and polymers which are irradiated with laser light and cross-linked by photolytic processes using specially developed laser systems which deliver ultra-short laser pulses to trigger processes that lead to polymerization in the volume. “This enables us to produce scaffolds for groups of cells with a resolution of approximately one micrometer directly from dissolved proteins and polymers to exactly match our construction plan”, explains Sascha Engelhardt, a project leader at the ILT.
Traditional “long runners” at COMPAMED are not only biocompatible materials, but also nanotechnology, which is gaining ever greater importance in medical applications. Material scientists at the University of Jena have developed a particularly successful combination of these two areas. Decisive factors for the success of biomaterials in the body are that they are not rejected, but that they function perfectly. A key factor for the acceptance is how endogenous proteins are taken up on the surfaces of implants. These human proteins also lubricate natural knee and hip joints, for example, by forming a protein layer over the cartilage. However, proteins are also used in artificial joints in order to reduce friction and resulting damage to the material. So far it has largely been unknown, however, how best to apply such proteins to artificial materials and what the ideal surface properties for this are. Ultra-high-molecular-weight polyethylene (UHMWPE) is used as a wear partner in artificial joints, which are usually made of metal or ceramic components. Dr. Thomas F. Keller from the Chair of Material Science at the Institute for Material Science and Technology (IMT) has discovered that proteins, which themselves are only ten nanometres in size, prefer to bond to nanocrystalline lamellae of UHMWPE. “The UHMWPE’s ability to align proteins by nanostructuring may be crucial to the friction properties of new joints ", says Dr. Keller, because joints such as knees or hips are often subjected to an unevenly distributed load by the patient’s own weight. “And although the joints do display a very complex wear pattern as we walk, the wear is basically unidirectional", he explains. “We now plan to transfer the new findings to other implant surfaces that make contact with the biological environment, so-called ‘biointerfaces’, here at the Chair of Material Science in Jena”, says professor Dr. Klaus D. Jandt. "Using molecular design on the nanoscale we aim to optimise other biological functions.” Not only does professor Jandt aim to minimise the wear of artificial joints, but also to optimise the tissue integration of implants.
Nanotechnology in the form of minute magnetic particles also plays a crucial role in a new medical imaging technique called Magnetic Particle Imaging (MPI), which has been developed by Philips (one of the exhibitors at MEDICA 2011) and which uses the magnetic properties of iron-oxide nanoparticles, so-called tracers. To generate the image, the tiny magnets are injected into the bloodstream. An MPI system is capable of detecting these particles spatially and quantitatively and is thus able to produce a 3D image of their local concentration, even in the course of physiological processes. This method of imaging has already proved capable of capturing precise real-time 3D images of blood flow and heart motion in pre-clinical trials. Now a consortium backed by the German Federal Ministry of Education and Research and with funding amounting to a total of €20.3 million aims to push research into the devices and tracers forward. “One of our key aims is to improve the size and shape of the particles, as the quality of the images depends on these factors. In addition to this, we also want to increase the yield of >good< particles to 30 – 60%”, explains Dr. Jörn Borgert, who is responsible for coordinating the research consortium at Philips. The benefits of the new technique include more thorough and faster examination with improved validity as well as the ability to do without the use of X-rays or radioactive materials.
Particular focus on precision and quality assurance
The product market “High-tech for Medical Devices” in Hall 8a, organised by IVAM, the professional association for microtechnology, is a firm fixture on the COMPAMED calendar. This year it will feature over 30 exhibitors and will focus primarily on the topics of precision and quality assurance. For example, 2E Mechatronic GmbH & Co. KG (Kirchheim unter Teck), which has been working on MID technology since 1998 (MID = Moulded Interconnect Devices), and is now also active in the field of medical technology, will be exhibiting at COMPAMED for the first time in 2011. Their latest product is a particularly high-performance MID LED light bulb that is produced using laser direct structuring (LDS). With this product, 2E has become the first manufacturer ever to use the LDS technique in medical technology. It took just six months of development work to be ready to go into production of a compact LDS-MID-based LED light bulb that offers users, for instance in the field of dental technology, significant advantages in comparison to conventional solutions (such as the high-pressure lamp). The LED light bulbs are far brighter and use less energy than conventional bulbs, while lasting up to six times as long.
To accompany the product market, IVAM and Messe Düsseldorf will also once again present the COMPAMED Forum (Hall 8a) this year, featuring high-ranking specialists who will give lectures and hold discussions for experts in the field of medical technology. “One of the highlights is the ‘Russia Session’ on day two of the fair“, says Orkide Karasu, fair project manageress at IVAM. Another forum on the topic of “supplier technology”, which makes its debut this year, is organised by the journal DeviceMed and will take place in Hall 8b. “Both of the forums are aimed at highly qualified specialists and leading experts from the medical supplies industry. Their lectures will provide information on new developments in the fields of materials, production techniques, nanotechnology as well as process control and global sourcing“, says Joachim Schäfer, Managing Director of Messe Düsseldorf, outlining the topics that will be covered.
COMPAMED 2011 will take place from 16 - 18. November in parallel to the world's largest medical trade fair, MEDICA (16 - 19 November 2011), with 4,500 exhibitors, in Düsseldorf. Of the 137,200 trade visitors that attended both events overall last year, more than 16,000 were interested in the specific range of topics covered by COMPAMED.
16.11.2011