Image source: Miyakawa et al., Nature Communications 2023 (CC BY 4.0)
News • Novel therapeutic strategy
Chemogenetics for the treatment of challenging epilepsy cases
Chemogenetics is a promising tool for treating neurological disorders by genetically modifying groups of neurons to make them reactive to specific drugs.
In a recent study, scientists from Japan demonstrated, for the first time, a successful chemogenetic suppression of widespread epileptic seizures in macaque monkeys. Their findings, which have been published in Nature Communications, represent an essential step towards clinical trials, taking us closer to an effective treatment for patients with severe epilepsy.
Epilepsy is one of the most common neurological diseases in the world. International health institutions estimate that over 50 million people worldwide are affected by it. Seizures, the hallmark symptom of epilepsy, are caused by abnormal and uncontrolled excitations of clusters of neurons. In severe cases, these excitations can spread across wide areas of the brain and trigger involuntary movements of the body and loss of consciousness.
We found that the anti-seizure effect of DCZ was not limited to the DREADD-expressing region, but instead extended to the entire region being recorded after the seizure had spread
Naohisa Miyakawa
On one hand, there has been remarkable progress over the past few decades on the treatment of epilepsy, enabling many patients to keep their symptoms under control. However, the drugs and surgical procedures typically used to treat epilepsy are known to interfere with normal brain function and cause undesirable side effects. Moreover, in certain cases, epilepsy can be resistant to drugs and even inoperable due to the affected neuron clusters being focalized in delicate areas.
These problems have prompted scientists to look for alternative therapeutic strategies involving new types of drugs. In the recent study, a research team led by Dr. Takafumi Minamimoto of the National Institutes for Quantum Science and Technology (QST), Japan, investigated the effectiveness of a promising approach based on chemogenetics that can potentially stop seizures on a dime. The team included Dr. Naohisa Miyakawa from QST, who is the first author of the paper.
But what is chemogenetics and how could it be the basis of a new therapy for epilepsy? Put simply, the main idea in chemogenetics is to alter a specific population of cells (such as neurons) to make them reactive to the presence of an otherwise unrecognized small molecule or drug. In neurology, a promising chemogenetic approach is based on DREADDs, which stands for “designer receptors exclusively activated by designer drugs.” When using DREADDs, the objective is to introduce carefully engineered receptors on the cell membranes of a target group of neurons. These receptors can only be activated by a specific drug that has no other biological effect. When the same drug is applied, the activity of the modified neurons can be quickly controlled thanks to the interactions between the receptor and the drug.
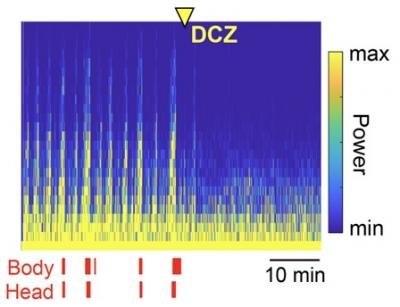
Image source: QST; Miyakawa et al., Nature Communications 2023
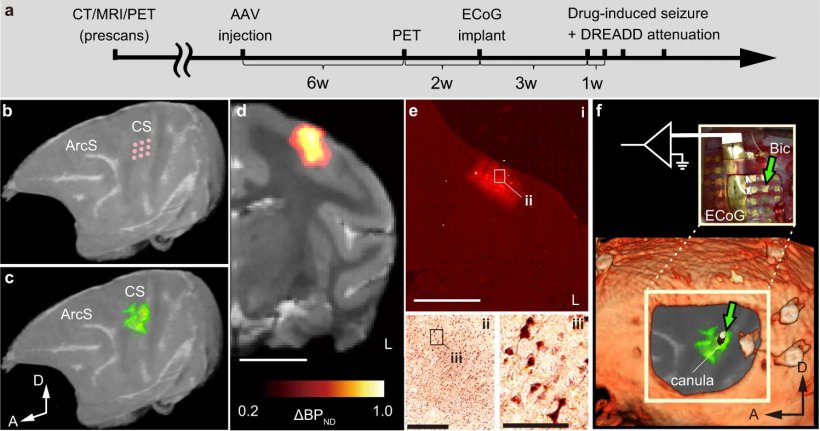
Based on this strategy, the research team tested a DREADD-based approach aimed at quickly attenuating the intensity of seizures. To this end, they conducted experiments on a species of macaque monkeys as a nonhuman primate model. By injecting a genetically modified virus directly into a specific region of their brain, they managed to modify the genetic material of a target group of neurons and forced them to express the DREADD receptor.
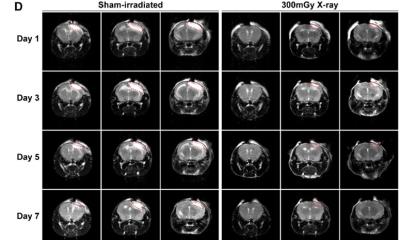
After confirming the presence of the receptor in the desired area using advanced imaging techniques, the team induced seizures in the brain of the monkeys by administering an drug that excites neurons. Next, to stop these seizures, they administered a DREADD-targeting drug, deschloroclozapine (DCZ), and monitored the response of the monkeys.
Overall, the results were remarkably good. Upon the application of DCZ, the induced seizures stopped within just three minutes. Most notably, DCZ was capable of keeping widespread seizures under control, highlighting its potential for treating severe epilepsy cases. “We found that the anti-seizure effect of DCZ was not limited to the DREADD-expressing region, but instead extended to the entire region being recorded after the seizure had spread,” remarks Dr. Miyakawa. “More specifically, DREADD/DCZ-induced inactivation in the seizure focus was enough to reverse seizures even after they had spread to both the brain hemispheres and had started producing involuntary body movements.”
Moreover, the proposed method caused no unexpected tissue damage or excessive inflammation, as well as no impairment of cognitive or motor abilities. These results certainly promise a bright future for DREADDs in the “on-demand” treatment of epileptic seizures. “To the best of our knowledge, this is the first successful proof-of-concept demonstration of chemogenetic seizure suppression in nonhuman primates, and represents an advancement towards clinical therapeutic applications,” highlights Dr. Minamimoto. “We plan to continue our research in collaboration with other research institutions and aim to apply this technology in clinical treatments, hopefully within the next 10 years,” he concludes optimistically. Additionally, other symptoms of abnormal neural activity, such as chronic pain, can be improved using a similar chemogenetic strategy.
With any luck, we could be on the verge of finally having a safe and effective treatment for managing epilepsy.
Source: National Institutes for Quantum Science and Technology, Japan
01.03.2023