News • Imaging
Accutron CT-D Vision - the new essential for contrast
With the launch of the Accutron CT-D Vision, MEDTRON AG once again demonstrates their status as a partner to radiologists. Focusing on the operator's needs, the latest evolution of the Accutron CT-D enhances the usability of its double head CT injector and optimizes its integration into the radiology environment.
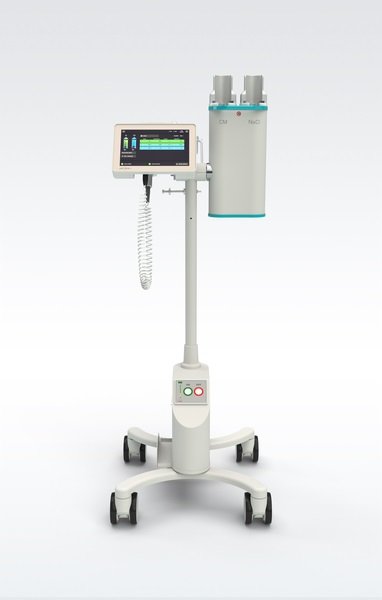
The modernized user interface makes the Accutron CT-D Vision even more user-friendly. It improves comfort, simplifies workflows and helps to improve patient care. Larger touchscreens on the injection unit and remote control increase readability and reduce eye fatigue.
Additionally, the Accutron CT-D Vision benefits from MEDTRON's intelligent Battery Management System, which optimizes battery charge duration and further increases operational availability. New medical-grade casters provide increased mobility by allowing the injector to be moved effortlessly and quietly around the exam room floor.
For optimal integration into the radiology environment, the Accutron CT-D Vision is also equipped with a new Injection Data Sharing (IDS) option. The IDS software option allows direct connection to RIS and PACS via a DICOM standard, enabling full traceability, documentation and analysis of contrast administration in computed tomography.
Source: Medtron AG
09.06.2021