Article • Automation, AI and more
Abundant ultrasound tech potential
Automation continues to conquer healthcare, including diagnostic imaging. Christian Kollmann, Assistant Professor at the Centre for Medical Physics and Biomedical Technology, Medical University Vienna, Austria, highlights innovative software, fast hardware and artificial intelligence in ultrasound – today and in the future.
Report: Marcel Rasch
Automated analyses are already supporting the diagnostic work-up. In Doppler ultrasound, for example, flow speeds are automatically detected and in b-mode grey-scale optimisation attenuation effects in deeper tissues areas are automatically adjusted by specific algorithms: one mouse click provides an overall homogeneous and smooth image.
Theoretically, constant velocity of ultrasound wave propagation is assumed; however, in reality velocity varies depending on the type of tissue, resulting in distortions and impaired resolution as can be seen inter alia in fatty tissue or large liquid-filled areas. Automatic adjustment of propagation above or below the theoretical value provides a solution to this problem. ‘This feature, which is offered by some manufacturers, is very much like autofocus in a digital camera,’ Kollmann explains. But beware: Despite the innovations, radiological experience is vital to be able to differentiate between software-induced artefacts and clinical findings.
AI is still in the early stages
This opens entirely new diagnostic perspectives, also for Doppler.
Christian Kollmann
Quantification and classification of tissue types, for example to recognise and characterise tumours, Kollmann says, is one of the areas where artificial intelligence is showing potential. ‘At this point, however, we are still in the research phase.’
Quantification and segmentation of organs face one major challenge: the visualisation of multi-layer areas with many different types of tissue. This complexity generates backscatter information leading to a wide variety of tissue parameters that need to be evaluated. Case in point: the abdomen. To be able to delineate and classify an organ unambiguously, the correct position data, dimensions and planes need to be determined. Moreover, echo amplitudes play a major role at tissue margins,’ Kollmann explains.
Superfast 3-D visualisation has three requirements: high-resolution, multiplex matrix transducers, fast data processing (state-of-the-art chip technology) and precise segmentation algorithms. ‘To be able to account for changes due to breathing, several hundred if not thousands of frames per second are required in a plane,’ Kollmann estimates. ‘This opens entirely new diagnostic perspectives, also for Doppler.’
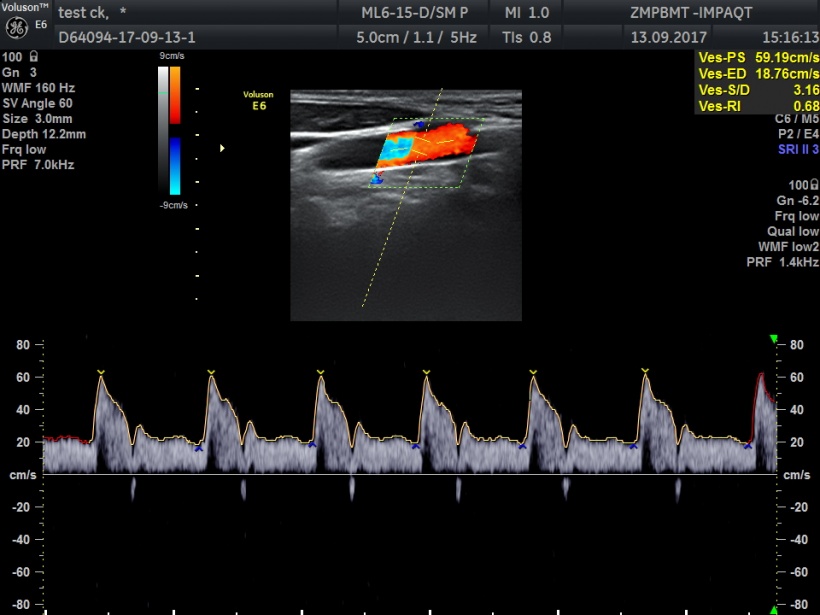
Diagnosticly valuable
Vector Velocity Imaging, an innovative diagnostic procedure, allows spatial and temporal 3-D visualisation of flow velocity. Complex blood flow patterns can thus be measured and diagnosed independent of transducer orientation.
A vector arrow indicates the direction of individual erythrocytes in the blood in a vessel. Due to the very high frame rates movement patterns, such as turbulences, can be recognised within milliseconds. The diagnostic relevance of this capability is obvious: critical changes can be detected in early stages. Kollmann is positive that ‘this technology will revolutionise ultrasound diagnostics and quantification in angiology and cardiology over the next five to ten years.’
Multi-parameters for classification purposes
To classify different tissue types and tumours even more precisely, additional quantification parameters are needed. One of the earliest attempts to do so was spectral-based parametrisation, invented several years ago to evaluate fatty liver. Today, elastography is the technology of choice for quantification. In shear wave imaging shear wave velocity is measured providing an additional parameter for the tissue type. Backscatter coefficient is another parameter, if it is available, adjusted for the propagation loss of the sound wave. Further parameters are being evaluated.
These multi-parameters are based on artificial intelligence algorithms and will allow the classification of tissue types. ‘This is the way forward,’ Kollmann believes. Combined, so-called hybrid procedures are also increasingly valuable: photoacoustics, for example, uses additional tissue parameters and today MRI-guided ultrasound is used for more than just uterine myoma interventions.
Ultrasound on mobile devices

New products link the wireless transducer to a tablet or smartphone via WiFi. These devices have enormous potential to teach and train physicians and sonographers, says Kollmann. The app is downloaded to an iPhone or Android tablet and training, using a miniature transducer, can begin. But this is not a toy, Kollmann underlines: ‘Actual patients should only to be used after instruction and in a controlled manner!’ After all, ultrasound exams do add energy to the organism, a fact that has to be taken into consideration even if this energy is harmless.
Point-of-care use of mobile devices is conceivable in, for example, an ambulance, car or helicopter. Transmission speed, according to Kollmann, is no major issue for tablets and smartphones even if, as announced, colour and Doppler devices with higher frame rates will be available. ‘This is entirely feasible using WiFi, as long as the signals are compressed,’ he points out. ‘The quality will no doubt be sufficient to visualise flow at the POC.’
Profile:
Christian Kollmann is Assistant Professor at the Centre for Medical Physics and Biomedical Technology at the Medical University Vienna, Austria. Having gained his doctorate in Technical Physics at Vienna’s Technical University, between 1990 and 2017 Kollmann – whose focus is ultrasound in medicine – published more than 140 articles in professional journals and gave more than 200 presentations on ultrasound and biomedical techniques.
14.11.2017