Article • Growing brain tumours in a petri dish
3D organoids for glioblastoma patients
Research that might lead to new treatment options and longer survival for patients with glioblastoma is ongoing at one of the largest and most modern facilities for brain tumour treatment in Germany.
Report: Brigitte Dinkloh

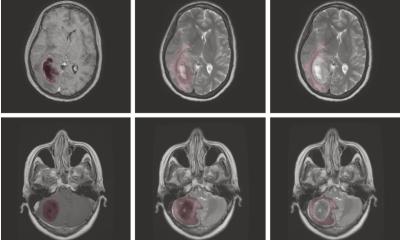
Glioblastoma are a malignant and particularly invasive type of brain tumour which is why ZHT, the Centre for Brain Tumours, and the Wilhelm Sander Neuro-oncology Treatment Unit at University Hospital Regensburg, have teamed up to develop effective strategies against it.
The hospital’s head of neuropathology, Professor Markus Riemenschneider, is a key member of the interdisciplinary clinical and research team, who studies the in vitro growth of glioblastoma tumour cells which behave like tumour cells in the human body. ‘These tumour organoids offer new insights into tumour growth. One day, we might even be able to translate this approach to personalised medicine and test for individual treatment response,’ Riemenschneider explained.
Maintaining variety in the cells

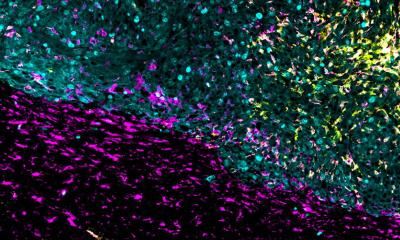
The Centre for Brain Tumours researchers have long been interested in molecular changes in cells. To start a cell culture, they use tissue samples that were isolated during surgery (with prior patient’s consent). Previously, this method had a major drawback: the cell heterogeneity could not be maintained – all cells were ‘harmonised’. ‘A typical feature of glioblastoma is cell heterogeneity,’ Riemenschneider pointed out. ‘There are different types of cells in a tumour, some are hypoxic, others are normoxic; that is, they differ in terms of oxygen concentration. Furthermore, you will see necrotic as well as perfused tissue areas. This variety of active tumour cells means a variety of genetic programs are running. When we isolate these cells and turn them into a cell culture, we only have one of these conditions – the variety is lost.’
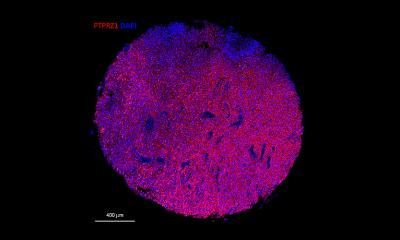
The new 3-D organoid models can overcome this hurdle. Currently, there are few studies on this issue – and these mostly on gastrointestinal and colorectal tumours. However, these models are crucial for glioblastoma research since all cells grow, interact and assume different functions – just like in a real tumour. ‘We use an extracellular matrix which provides a surface for these cells to dock on. The matrix is metabolised after a while, but the cells retain their structure and integrity. This is promising because it might enable us to model the actual patient situation more realistically: when we can map the gradients, for example regarding oxygen saturation and nutrient supply, we will be able to see much more than in a normal cell culture,’ the neuropathologist added.
Producing a brain
Tumour organoids are limited neither by species barriers nor artefacts – two obstacles in research that uses tumour cells implanted in mouse brains. Moreover, interindividual barriers don’t exist in a purely autologous system. The researchers, who cooperate with colleagues at the University of Erlangen, are not only able to produce tumour organoids in a Petri dish; they can even produce miniature brains, using a procedure which received the 2012 Nobel Prize for medicine: all cells of the human body can be produced from healthy cells via induced pluripotent stem cells.
‘This procedure,’ said Riemenschneider, ‘opens up entirely new options to confront cultured patient tumour cells with own miniature brains. This allows us to observe tumour cells’ growth onto and into the brain cells – this is what, de facto, determines healthy and pathological cells. In the long run, we hope to develop treatment options.’ The research project is still in an early phase, but the goal is a standardised procedure that tests treatment response and effectiveness on the individual patient level.
Insights may come into rare brain tumours

In turn, this might yield insights relevant to the treatment of rare brain tumours that are difficult to assess in conventional cell cultures. Since there are more than 100 types of brain tumours, a highly differentiated approach is needed.
The Wilhelm Sander Treatment Unit appears to be heading in the right direction as shown in the enhanced patient survival rate recorded since the facility was set up. Whilst overall glioblastoma prognosis remains poor, at the centre we could significantly prolong average survival rates. ‘We aim to ensure a longer good quality life for our patients,’ Riemenschneider stressed. With his Regensburg team, he has taken the first successful steps towards this goal: two to three months post-surgery tumour organoid cultures are available for certain patients. These can be used to test treatment response in the lab should a tumour return.
Profile:
Dr Markus Riemenschneider, a University Professor and Head of Neuropathology at the University Hospital Regensburg since 2010, received his medical training as well as specialist training in neuropathology at the University of Düsseldorf. A Mildred Scheel Scholarship of the Deutsche Krebshilfe Foundation enabled him to become a researcher in the Department of Pathology at the Massachusetts General Hospital and Harvard Medical School, Boston, USA. In 2008, he completed his specialist physician training in neuropathology and headed the independent Max-Eder Junior Working Group of the Deutsche Krebshilfe in Düsseldorf until his present appointment in Regensburg. Riemenschneider has received a number of awards for molecular neuro-oncology research, and was, inter alia, appointed a member of the Junge Kolleg (Young Scholars Group) of the North Rhine-Westphalian Academy of Sciences, Humanities and the Arts. In 2011, he received the Sibylle Assmus Award for neuro-oncology. Another focus of the professor’s work is quality control in neuropathology. He is an expert for DAkkS – the national accreditation body for the Federal Republic of Germany – and a member of the Scientific Board of QuIP GmbH – Quality Assurance Pathology Initiative GmbH, in Berlin.
31.10.2019