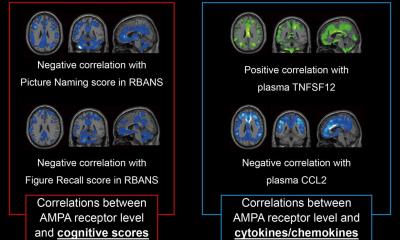
Developments in molecular evidence-based medicine and imaging
By Johannes Czernin MD, Professor of Molecular and Medical Pharmacology, Chief, Ahmanson Biological Imaging Division, Department of Molecular and Medical Pharmacology, David Geffen School of Medicine at UCLA
In the current economic environment the introduction of novel imaging approaches and their reimbursement by payers is becoming increasingly difficult. Historically, this denotes a significant change, in that many currently accepted routine tests or interventions were accepted based on common sense, convincing experience or rapid adoption into clinical routine without much scrutiny.

Some examples of such acceptance based on empiric evidence include the adoption of intravenous contrast for CT, MRI and ultrasound imaging, or the rapid utilisation and reimbursement of organ transplantation or coronary artery bypass surgery.
As another example the RECIST (Response Evaluation Criteria in Solid Tumours) criteria for determining tumour responses to treatment have been adopted worldwide, despite the near complete lack of evidence that these criteria help to identify those patients who benefit from sometimes highly toxic treatments. At a minimum, this approach identifies treatment responses only late after completion of therapy and legions of cancer patients are frequently exposed to inefficient therapies.
Due to increasing healthcare costs a more objective evidence based approach has been promoted and frequently has been implemented. While of unquestionable value in some instances, this approach also can lead to marked delays in the clinical introduction of novel imaging technologies, or sometimes life-saving surgical or medical interventions.
In 1996, Sackett et al defined evidence-based medicine as ‘the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients’. Several ‘levels of evidence’ have been proposed whereby the highest level of evidence would arise from at least one properly designed randomised trial. For example, this definition applies well to measuring drug effectiveness but has considerable limitations when applied to imaging.
Let’s assume one wants to determine the diagnostic accuracy of an imaging test: the best way to determine its diagnostic performance would be a direct comparison between the two modalities relative to a gold standard. This of course cannot be done in a randomised test model. If, however, another layer of complexity is added, such as, for instance, by studying the impact of a test on subsequent healthcare utilisation, or even patient outcome, then a randomised test would be needed. Thus, demanding level one evidence for acceptance/reimbursement of an imaging test appears to be a mindless exercise in futility.
Evidence-based medicine is a useful model for cost containment in healthcare. However, it carries substantial risks of denying patients the best care that can be evident from empirical data.
Thus, imaging studies addressing different question demand various study designs that are frequently not consistent with the definitions of evidence-based medicine. For instance, when diagnostic accuracy is the issue randomised trials are not only useless, they are in fact irrelevant and can frequently not provide the correct answer. If the impact of tests on patient management and outcome is in question, then randomised designs are useful and necessary.
The United States Centres for Medicare & Medicaid Services (CMS) has put in place an interesting model requiring evidence that any test needs to demonstrate its impact on patient management prior to coverage decisions. This has led to the large National Oncology PET Registry (NOPR), a registry that measured the impact of FDG PET imaging on management decisions. The data obtained from this registry demonstrated that FDG-PET impacts on treatment decisions in more than 30% of all cancer patients. Based on these findings CMS coverage for FDG-PET in cancer imaging was expanded in the USA. Since this decision, CMS has further raised the level of required evidence. In a recent decision, CMS has concluded that imaging tests should only be reimbursed if outcome of patients is improved by the introduction of the test. The difficulty of this new demand is evident. If for instance a novel imaging probe for detecting bone metastases is introduced a randomised trial would be designed to show superiority/inferiority of the test with regards to accuracy. However, thousands of patients would need to be enrolled to show an impact on patient outcome.
The public rightfully demands a rapid translation of discoveries in diagnostics and therapeutics into the clinic. Regulatory agencies are challenged to enable such rapid translation.
06.03.2010