Article • Molecular Imaging
Xe-MRI advances body exploration
Clinical routine would be inconceivable without MR Imaging. Without exposure to radiation, doctors can make a patient’s organs and tissue structures clearly visible. However, pathological changes in the early stages, degenerated cells or small areas of inflammation, have so far remained almost invisible on these images. In 2014, for the first time, a team of cell biologists, chemists and physicists working with Dr Leif Schröder, Head of the ERC-Project on Biosensor Imaging at the Leibniz-Institute for Molecular Pharmacology (FMP) in Berlin-Buch, succeeded in generating ‘two-colour’ images for different molecular markers with the help of Xenon-MRI.
Report: Sascha Keutel

Xenon-MRI (Xe MRI) was developed in the mid-1990s to facilitate diagnostic imaging of the lung. Unlike conventional MRI it does not detect water molecules but instead the non-poisonous, noble gas Xenon. The system then directly visualises the ventilated lung areas. For new kinds of imaging, first a contrast medium is administered, which binds specifically to the marker and accumulates in the diseased tissue. Then Xenon is administered, either via inhalation of a mixture of gases containing Xenon, or dissolved in substances such as blood plasma. Advantage: These directly injectable substances can be available in the blood stream immediately.
The FMP scientists aim to bind Xenon gas combined with biosensors, like a contrast medium, to go precisely to disease-specific markers in the tissue. ‘We very recently published the results of a first study where we could bind Xenon biosensors to certain glucose structures on the cell surface. These play a certain role in tumours and cannot be visualised with other MRI contrast media. This was proof that the technology has made structures visible for the first time that were previously inaccessible via MRI,’ Schröder reports.
We very recently published the results of a first study where we could bind Xenon biosensors to certain glucose structures on the cell surface.
Leif Schröder
The scientists also succeeded in marking different cell types so that they send out radio waves on different frequencies. In the same way as with a light microscope they generate images where some cells glow in red, others green. The researchers used a concept from the world of laser physics where the Xenon signal is amplified about 10,000 times – the gas is ‘hyperpolarised’. In the test object itself they then rescind this situation in a controlled manner through a co-action between the biosensor and the MRI exposure sequence.
This results in a further amplification, with an order of magnitude of around three, and allows the detection of relatively small amounts of the specific marker. In the first step, biosensors without Xenon are administered that selectively bind to diseased cells or, respectively, are washed out in other locations. Once the Xenon has dissolved in the nutrient solution for the cells – or later in the patient’s bloodstream – the bound sensors become visible and show the sites of pathological changes. Then the Xenon is detected; in the MRI it ‘gives itself away’ through a certain resonance frequency in the MRI when bound in the sensors. The images are then superimposed with conventional MRI images to obtain anatomical as well as biochemical information (such as in the PET/MRI hybrid procedure).
Xe MRI has various fields of application
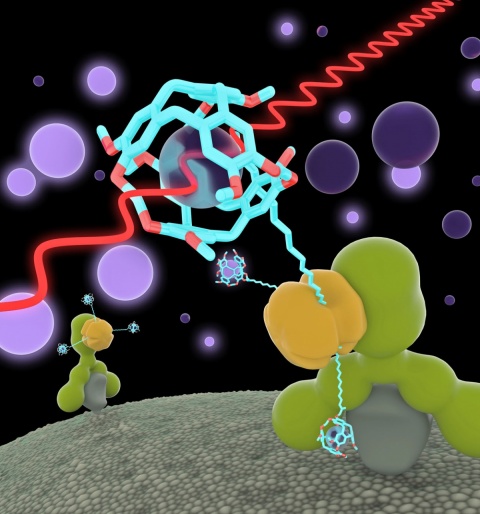
With the help of Xe MRI the researchers aim to significantly expand the range of applications for MRI. They are convinced that, in the future, the refined procedure will facilitate more precise diagnosis because the stronger signals can visualise structures not previously detectable in the MRI. ‘As a noninvasive procedure with excellent soft tissue contrast and no radiation exposure the only entity missing is the molecular specificity to turn MRI into the ideal procedure,’ the medical physicist believes.
The method is also very important for the development of active ingredients. He hopes it will help to reduce the number of animal experiments required and to assess the effectiveness of new active ingredients quickly.
Although the team’s current research focuses on oncology diagnostics Xe MRI is not limited to any particular type of disease. ‘Everything that has an identifiable molecular marker can, in principle and at sufficient concentrations, be detected with biosensors. ‘Currently the detection limit is in the nanomolar range, making these markers around 1,000 times more sensitive than conventional MRI contrast media. ‘We have developed sensors for inflammatory processes and for certain receptors on the surface of cancer cells, but we can build on this in a very flexible way,’ the physicist points out.

Whether or not Xenon-MRI will become established in clinical routine is currently difficult to assess. The first trials on animal models are planned for this year, but Schröder cautions: ‘It’s likely to take several years before the procedure can be used in clinical routine. However, patients will also benefit indirectly from the use on animal models, which is expected to begin much sooner, through the improved development of new active ingredients and treatments.’
Profile:
Medical physicist Leif Schröder has led the Molecular Imaging research group at Leibniz Institute for Molecular Pharmacology (FMP) in Berlin since 2009. Their focus is on the development of Xenon biosensors that highly increase the significance of magnetic resonance imaging (MRI). Schröder gained an initial five-year grant from the European Research Council to explore the potential of these novel contrast agents. Among the physicist’s many awards are the Emmy-Noether research fellowship and the Philips Research Prize for medical physics.
06.03.2015





