Source: Royal Philips
News • FDA clearance
Ultrasound for COVID-19-related lung and cardiac complications
Royal Philips has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market a wide range of its ultrasound solutions for the management of COVID-19-related lung and cardiac complications. Handheld and portable ultrasound solutions in particular have become valuable tools for clinicians treating COVID-19 patients due to their imaging capabilities, portability and ease of disinfection.
With this regulatory clearance we can offer clear guidance to ensure safe and effective use of ultrasound to manage COVID-19-related lung and cardiac complications.
Bich Le
As a result of this regulatory clearance, which is an industry first, Philips can provide detailed, practical guidance to support clinicians using its systems and software for patients affected by COVID-19. The clearance applies to Philips ultrasound systems including the EPIQ series, Affiniti series, Lumify, CX50 and Sparq diagnostic ultrasound systems, and to off-cart solutions like QLAB Advanced Quantification Software.
Ultrasound has shown value in imaging peripheral lung tissue affected by pneumonia, which is closely tied to COVID-19 lung complications. As respiratory strain can also lead to cardiac dysfunction, COVID-19 patients are at increased risk for cardiac complications. A cardiac ultrasound exam can help in evaluating the effects that disease progression may have on heart function. By imaging COVID-19 patients at the point of care, such as in the Emergency Department (ED) or Intensive Care Unit (ICU), clinicians can diagnose and monitor patients without the need to move them around the hospital, helping to reduce the risk of virus transmission to other patients or to healthcare professionals.
First ultra-portable ultrasound device with advanced telehealth capabilities
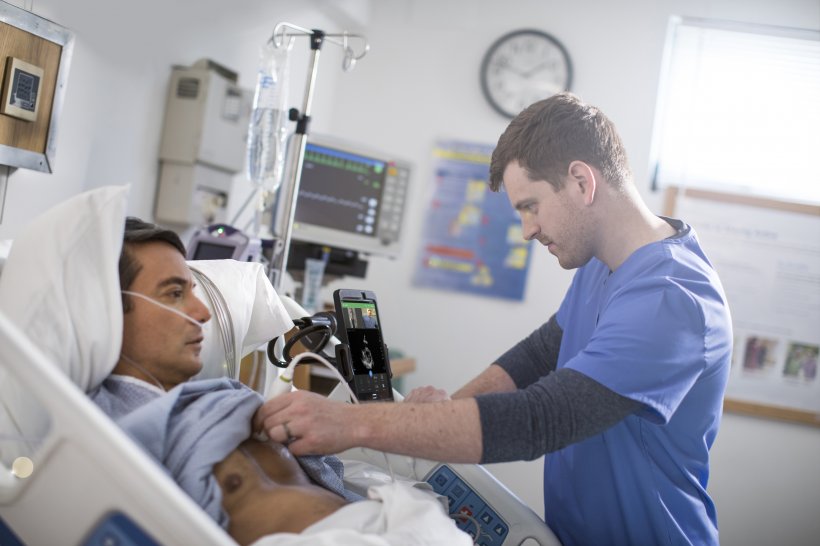
“Many healthcare providers have told us that our handheld and portable ultrasound solutions are playing a valuable role in their efforts to combat COVID-19,” said Bich Le, Senior Vice President and General Manager Ultrasound at Philips. “With this regulatory clearance we can offer clear guidance to ensure safe and effective use of ultrasound to manage COVID-19-related lung and cardiac complications. At the same time, we are investing significantly to ramp up production globally, including at our ultrasound manufacturing plants in the US.”
With its broad portfolio, leadership in areas including cardiac ultrasound and the unique capabilities of the Lumify with Reacts handheld tele-ultrasound solution, Philips is well positioned to support healthcare providers with ultrasound solutions as they combat the pandemic. The Lumify with Reacts point-of-care ultrasound solution, which works in conjunction with a compatible smartphone or tablet, is the world’s first ultra-portable ultrasound device with advanced telehealth capabilities. The Reacts communications platform enables two-way audio-visual calls with live ultrasound streaming, so both parties can simultaneously view the live ultrasound image and probe positioning, while discussing and interacting at the same time. In the context of COVID-19, this solution can help minimize the risk of virus transmission for the medical team.
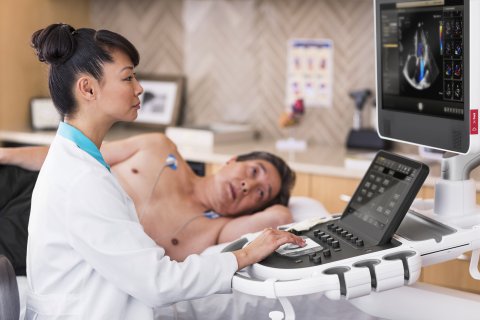
The new guidance highlights the specific presets, transducers, quantification tools and other capabilities available on Philips’ ultrasound systems that are relevant in assessing and managing COVID-19-related lung and cardiac complications. For example, the EPIQ CVxpremium cardiology ultrasound system includes automated applications for 2D assessment of the heart, as well as robust 3D right ventricle volume and ejection fraction measurements.
The regulatory clearance includes the following Philips ultrasound systems: EPIQ series, Affinitiseries, Lumify, CX50, and Sparq diagnostic ultrasound systems and off-cart solutions like QLABAdvanced Quantification Software. More information on how Philips is responding to COVID-19 can be found on the company’s global newscenter.
Source: Royal Philips
15.05.2020