Interview • Lifestyle tests
The dangers of commercial direct-to-consumer tests
Lifestyle tests that pretend to be medical procedures are inherently problematic in terms of clinical, medical and privacy issues.
Integration of DTC testing

Professor Matthias Orth, Medical Director of the Institute of Laboratory Medicine at Marienhospital Stuttgart, discussed ‘Direct-to-consumer testing: The business of lifestyle tests’ at a September congress in Copenhagen that focused on ‘The benefits and challenges of point-of-care testing across the clinical spectrum’.
Direct-to-consumer testing (DCT), or direct access testing (DAT), are laboratory tests with results that go straight to the end customer or patient, explained Professor Matthias Orth. ‘This includes tests ordered by a physician for medical purposes and clearly evidence-based tests, such as glucose monitoring or blood glucose sticks for diabetes patients. These exams are also POCTs – point-of-care tests. Furthermore, DTC tests encompass test strips, for example urine pregnancy tests or lactate tests in your gym.
‘However, more recently a new generation of DCT tests has emerged: tests where the customer samples blood or DNA at home, mails the sample to the lab and then calls up results over the internet. According to the test providers, each sample is individually analysed, with the test pretending to be a medical lab service.’
Are these tests a problem for lab specialists and other healthcare professionals?
‘In most countries the healthcare sector is highly regulated to protect the patient. In the USA, for example, the tests need FDA approval and labs must be certified by the Clinical Laboratory Improvement Amendments (CLIA). In Germany, we have a guideline issued by the German Physicians’ Association for Quality Assurance of Lab Medical Exams. According to this, tests must offer a medical benefit; moreover only adequately qualified people can perform tests.
‘There are also many more rules and regulations to protect the patient, such as privacy laws, regulations applying to healthcare professionals, the genetic diagnostics law and rules that govern physicians fees for services.
‘All these – undoubtedly necessary and useful – laws and rules do not apply to lifestyle testing. Therefore we must now somehow define when the line between lifestyle and healthcare is crossed and when legal action is required. That is very difficult, as we saw recently when the business model of Theranos, the worldwide largest provider of DTC testing, turned out to be a complete fraud. Scientists and physicians alike had relied on Theranos methods. It was claimed that there is a conflict of interest, because they themselves provide diagnostic services and there were attempts to force them to cease and desist from using the Theranos methods.
‘It is also highly problematic when physicians use results from lifestyle DTC tests since this basically means that lifestyle test results are jazzed up to be healthcare diagnostic results – but these lifestyle tests follow no legal and technical standards whatsoever. If the patient suffers damages, the physician who accepted the DTC results is liable even though he probably was not involved in the generation of the results at all.’
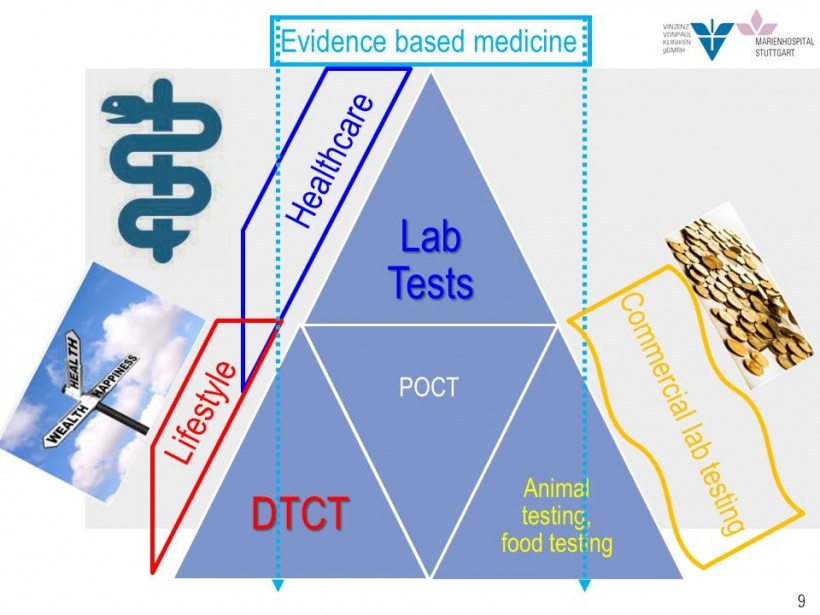
Do these tests impact on work in the lab?
‘One important danger I see is the careless handling of medical data in DTC testing. There is high potential for abuse, particularly with molecular exams. It defeats me how people can thoughtlessly allow 23andMe, a genetic testing company with close links to Google, the use of their genetic and other highly sensitive data.
‘Another problem is the waste of healthcare resources through DTC testing: The business model of DTC testing often entails the fabrication of conspicuous findings in order to sell, for example, dietary supplements, or expensive monitoring services. Thus lifestyle tests often lead to complex diagnostic procedures of a perfectly healthy person – with the general public footing the bill.
‘A third issue is the media hype surrounding DTC testing, something we have never seen in evidence-based medicine: DTC testing services are regularly promoted in blogs, user groups and the social media. The promotional texts, even though they might be labelled as “advertisement” somewhere, convey images of a certain lifestyle and make claims regarding DTC testing that are in no way supported by evidence. However, we find it impossible to counter these internet trolls with reasoned arguments.’
Should DTC be more strongly regulated?
We do need the active support of all lab specialists in order to contain this uncontrolled growth.
Prof. Matthias Orth
‘The definitions of DTC testing are indeed very complicated and we do see efforts of DTC testing providers to circumvent the healthcare rules and regulations that are in place and necessary to ensure patient safety. Think of the over-the-counter sale of lab tests in pharmacies, or the call for tenders for genetic testing under section 140a of the German Social Code, book V regarding special services.
‘Moreover, internet offerings and mail order pharmacies cross national boundaries and thus turn many reasonable regulations into blunt instruments. In addition, DTC testing providers legitimise their services by referring to the EU consumer rights directive 2011/83/EU, which is meant to promote free movement of goods and services.
‘In my opinion that is dangerous and very naïve: Today, even small forensic DNA specimens provide information on features as such ethnic background, size, hair colour, etc. It is naïve to assume that complete genome sequencing in a lifestyle test done for genealogical purposes does not provide medical data that need to be protected. Consumer protection in DTC testing is marginal. The limitations of the methods are hidden in the small print and difficult to understand.
‘We do need the active support of all lab specialists in order to contain this uncontrolled growth.’
Is Europe’s situation different in the USA?

‘While the overall objective, to protect lab diagnostics, is similar across countries, the legal frameworks differ considerably. In Europe genetic data enjoy particular protection whereas in the USA’s lifestyle tests are not per se prohibited. Moreover the rules to ensure quality standards in the labs differ widely. In Germany, for example, quality assurance is concerned primarily with structural quality; in the US it focuses on the quality of the results.
‘It’s crucial that every country views assurance of patient safety as the interplay of different rules and regulations. We must not undermine very successful quality assurance and patient safety structures by pointing to isolated rules in other countries.’
Profile:
Professor Matthias Orth MD gained his doctorate in 1994 at Albert Ludwig University, Freiburg, Germany. Following specialist courses in clinical chemistry, internal medicine, microbiology and immunology, he worked at the Institute of Clinical Chemistry and Pathobiochemistry at Freie Universität Berlin. From 2000 to 2003 he was Managing Senior Physician at the Institute of Laboratory Medicine, Clinical Chemistry and Molecular Diagnostics at University Hospital Leipzig, gaining his habilitation in 2004. He then became Medical Director of the Institute of Laboratory Medicine at Marienhospital Stuttgart. Orth is a Board Member of the German Society for Clinical Chemistry and Laboratory Medicine, Chairman of the Laboratory Management Section at DGKL and a Board Member of the German Professional Association of Laboratory Medicine.
20.02.2017