Article • Surgery
The 3-D printing revolution
‘It’s cheap, it’s accessible, and 3-D printing helps students to rapidly try out their product ideas,’ said Robert Webster, a visiting speaker at TU Delft from Vanderbilt University School of Engineering.
Report John Brosky
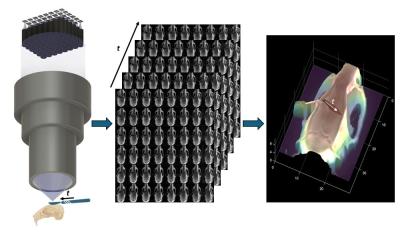
At the Delft Technical University they call themselves the Bio-Inspired Technology Group, or BITE. And their claim to fame is in having created DragonFlex, the world’s first steerable surgical instrument made entirely by 3-D printing.
While the prototype is more likely to earn doctoral degrees for the inventors than to win a CE certification for use by surgeons, it demonstrates the emerging robustness for 3-D printing technology.
‘It’s cheap, it’s accessible, and 3-D printing helps students to rapidly try out their product ideas,’ said Robert Webster, a visiting speaker at TU Delft from Vanderbilt University School of Engineering.
‘As the prices come down dramatically and performance goes up, it’s reasonable to think that in a few years, the idea of a hospital having a 3-D printer in the basement to create on demand patient-specific implants or customised instruments for surgery, is absolutely feasible,’ he said. ‘The hospital could take any scan, print it and create a 3-D model.’
According to Amir Zadpoor, Director of the Additive Manufacturing Lab at TU-Delft, when rapid prototyping using 3-D printers first became feasible, academic projects made up 80 percent of the activity.
Device designers in industry quickly caught up, calling it ‘additive manufacturing’ instead of 3-D printing. While this year more than $3 billion will be spent as companies find new applications to convert traditional manufacturing processes, this investment is expected to reach $20 billion annually in just a few more years.
Zadpoor notes that medical devices today account for 40 percent of that spending ‘… because there is such a great added value to these products that justify the increased cost associated with the technology’.
The pioneer in additive manufacturing applied to orthopaedic devices is Warsaw, Ind.-based Zimmer-Biomet Inc., which began developing products 15 years ago.
Using what is called a build plate, industrial 3-D printing involves building up micro layers of titanium powder that are burned with a laser to solidify the powder into a metal with nano-precision to match the design model.
By the end of 2015 manufacturers had installed around 300 machines for 3-D printing of implantable prostheses.
Whereas in 2010 there were just four 3-D-printed implantable devices approved by the United States Food & Drug Administration, by 2014 twenty-five products had been FDA approved.
According to Kevin Lobo, CEO at orthopaedics leader Stryker Corporation, additive manufacturing is ‘having an impact on our knee business as well as spine, and we have a huge line up of other divisions with ideas and prototypes to get into 3-D printer titanium product’.
In 2016 Stryker began construction of its second 3-D printing facility in Cork, Ireland.
Orthopaedics market specialist Ali Madani, from Avicenne Medical in Paris, said that titanium spinal cages made by additive manufacturing processes constitute the most dynamic orthopaedic segment and that these products are steadily eroding the market share for polymer-based PEEK cages.
In Europe, Madani pointed out that Italy is home to the most advanced companies in additive manufacturing where challengers like Lima Corporate, based in San Daniele del Friuli, and Milan-based Adler Ortho have invested massively in the technology and each year sell thousands of 3-D printed hip cups, shoulder implants, knee tibial plates, or mini-hip stems.
Robin Stamp, the associate manager for Advanced Technology at Stryker Orthopaedics explained that for over 20 years, Stryker has milled and machined metal implants using complicated, multi-step manufacturing processes based on coating materials with rigid requirements that limit design options.
‘What 3-D printing does is give design freedom, an ability to try exotic designs, build channels into the surface, create roughness, give a product any feature needed for essentially the same cost as building a standard model,’ he said. ‘Where we are really seeing a difference is in the speed of design iterations.’
Instead of a product development cycle of 18 to 24 months with a high cost for making changes, he said that today, using additive manufacturing, his group can produce a design and within one week, give the part to a panel of surgeons and rapidly iterate, based on the panel’s suggestions to further develop the design.
‘This is phenomenally powerful,’ he said. ‘We are capable of doing so many more iterations, putting much more functionality and creativity into products.’
30.03.2017