News • Rare bone cancer
Targeted approach to therapy for chordomas
Chordomas are rare bone tumors for which only few options of treatment exist. Scientists and doctors from the National Center for Tumor Diseases (NCT), the German Cancer Research Center (DKFZ), and Heidelberg University Hospital (UKHD) have discovered a particular genetic trait of chordomas in advanced stages after conducting gene analysis.
Their findings, published in the journal Nature Communications, indicate that a group of drugs, which have already been approved for treating other types of cancer, could also be effective in treating chordomas.
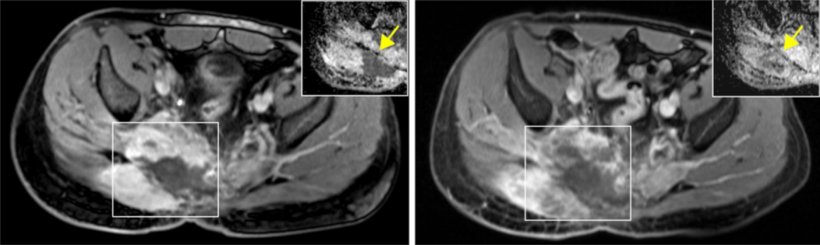
Credit: NCT Heidelberg
Chordomas are rare tumors of the spinal column. They make up around one percent of all bone tumors. They are categorized as bone tumors; however, they do not develop in bone tissue but rather from remnants of the so-called chorda dorsalis, which is found on the end of the spine. Usually this tumor of the spinal column appears in patients after they reach 30 years of age, and it affects men and women equally. Treatment is often difficult because chordomas are typically resistant to conventional chemotherapy. Doctors try to surgically remove the affected tissue, which is not always completely successful. As a result, the majority of those affected must subsequently undergo radiotherapy. In about two thirds of cases, the tumor comes back again. Researchers are therefore looking for new ways and approaches to deal with this disease.
Scientists and doctors from NCT Heidelberg, DKFZ, and UKHD in cooperation with colleagues from the German Cancer Consortium (DKTK) have now completed a detailed gene analysis of tumor cells from chordoma patients. The project was part of the NCT/DKTK MASTER (Molecularly Aided Stratification for Tumor Eradication) program. The study headed by Stefan Fröhling, Managing Director of NCT Heidelberg, addresses especially young patients with advanced-stage cancers and patients with very rare tumors.
In chordomas, apparently other, hitherto unknown genetic changes lead to an impairment of homologous recombination (HR)
Stefan Gröschel
Overall, the team examined eleven patients with chordomas in an advanced stage and who had already undergone a wide range of standard therapies. The scientists completely sequenced the genetic material in the cancer cells and discovered that advanced-stage chordomas exhibit certain molecular changes associated with an abnormal DNA repair via a process called homologous recombination (HR).
In general, cells use HR to repair damaged portions of DNA strands. It is well known that HR is impaired in other types of cancer cells. Certain conditions must be in place to correctly diagnose this incorrect function. "However only three of the eleven patients studied showed these criteria," reports Stefan Gröschel, Attending Physician at UKHD and Head of the Research Group Molecular Leukemogenesis at DKFZ. "In chordomas, apparently other, hitherto unknown genetic changes lead to an impairment of HR."
As certain drugs have proven to be effective in other types of cancer that also demonstrate a lack of HR, they appeared as a good option for additional treatment of chordoma patients. The doctors conducted experimental treatment using a PARP inhibitor in an individual patient with the matching genetic profile. PARP inhibitors inhibit the enzyme poly-ADP ribose polymerase and prevent the cancer cells from repairing damage to their DNA, which may occur after chemotherapy. After administering a PARP inhibitor, the patient demonstrated a long-lasting clinical improvement, and the tumor stopped growing. "Our results show how the search for new personalized cancer treatments can be applied in clinical practice." By administering an approved drug that had yet to be used to treat chordomas, we were able to improve the patient's condition over a period of ten months," reports Fröhling.
In addition, the team led by Fröhling, Gröschel, and Robert Russell from Bioquant Heidelberg was able to identify a new resistance mutation of the PARP1 enzyme, which counteracted the effect of the PARP inhibitor in the same patient after the disease once again showed signs of progression.
Source: National Center for Tumor Diseases (NCT) Heidelberg
09.04.2019