News • Lipid research
Solving the sub-zero challenge of Covid-19 vaccines
New research by University of Texas at Dallas scientists could help solve a major challenge in the deployment of certain Covid-19 vaccines worldwide — the need for the vaccines to be kept at below-freezing temperatures during transport and storage.
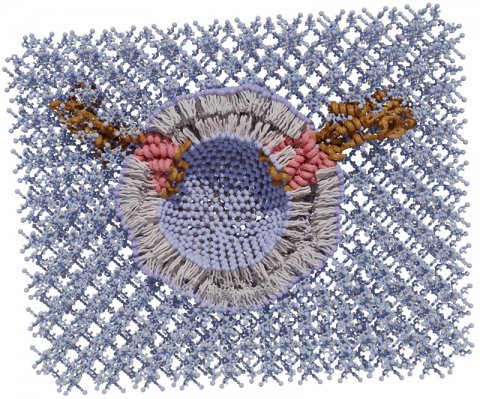
Image source: University of Texas
In a study published online in Nature Communications, the researchers demonstrate a new, inexpensive technique that generates crystalline exoskeletons around delicate liposomes and other lipid nanoparticles and stabilizes them at room temperature for an extended period — up to two months — in their proof-of-concept experiments.
The Moderna and Pfizer/BioNTech Covid-19 vaccines use lipid nanoparticles — basically spheres of fat molecules — to protect and deliver the messenger RNA that generates a vaccine recipient’s immune response to the SARS-CoV-2 virus. “The expense of keeping these vaccines very cold from the time they’re made to the time they’re delivered is a challenge that needs to be addressed, especially because many countries don’t have sufficient infrastructure to maintain this kind of cold chain,” said Dr. Jeremiah Gassensmith, associate professor of chemistry and biochemistry and of bioengineering at UT Dallas and a corresponding author of the study. “Although we did not include in this work the specific lipid nanoparticles used in current Covid-19 vaccines, our findings are a step toward stabilizing a lipid nanoparticle in a way that’s never been done before, so far as we know.”

Image source: University of Texas
The idea for the research project began during a coffee-break discussion between Gassensmith and Dr. Gabriele Meloni, a corresponding co-author of the study and assistant professor of chemistry and biochemistry in the School of Natural Sciences and Mathematics at UT Dallas.Gassensmith’s area of expertise is biomaterials and metal-organic frameworks, while Meloni’s research focus is transmembrane transporter proteins. These proteins reside within cell membranes and are crucial for moving a variety of small molecules, including ions and trace metals, in and out of cells for several purposes. “Membrane proteins sit in a cell membrane, which is a lipid bilayer,” Meloni said. “To study their structure and biophysical and biochemical properties, we must extract these proteins from the membrane using detergents and then reconstitute them back into an artificial membrane — a proteoliposome — that mimics the proteins’ natural environment.”
Lipid nanoparticles and liposomes are similar in structure, and neither are thermodynamically stable at room temperature, Gassensmith said. The lipid structures can fuse or aggregate, exposing any embedded membrane proteins or cargo to degradation. “One of the challenges in my field of research is that both membrane proteins and lipid bilayers are very delicate and intrinsically metastable, and we’re trying to combine them in order to understand how these proteins function,” Meloni said. “We have to handle them carefully and prepare them fresh each time. They cannot be stored for long periods and are not easily shipped to colleagues in other labs.” The researchers joined forces to develop a methodology to stabilize this kind of lipid system and demonstrated their results using transmembrane proteins from Meloni’s lab as a case study.

Image source: University of Texas
They mixed liposomes — some with embedded proteins, some without — with a combination of two inexpensive chemicals, zinc acetate and methylimidazole, in a buffer solution. In about a minute, a crystal matrix began to form around individual liposomes. “We think that the lipids interact with the zinc just strongly enough to form an initial zinc-methylimidazole structure that then grows around the lipid sphere and completely envelops it, like an exoskeleton,” Gassensmith said. “It’s analogous to biomineralization, which is how certain animals form shells. We sort of co-opted nature in creating this totally fake shell, where the biomacromolecules — the lipids and proteins — catalyze the growth of this exoskeleton.”
The ability of biomimetic shells to form around biological molecules is not new, Gassensmith said, but the process hasn’t worked well with lipids or liposomes because the metal salts that comprise the shell material suck water out of the liposomes by osmosis and cause them to explode. “One of the keys to this research was identifying the buffer solution in which everything resides,” Gassensmith said.
Three graduate students collaborated on the project to develop the unique buffer medium that allows the reaction to occur. “The buffer medium maintains the ionic strength of the solution and keeps the pH stable so that when you add a huge amount of metal salts, it doesn’t osmotically shock the system,” said Fabián Castro BS’18, a chemistry doctoral student in Gassensmith’s lab and a lead author of the study. Castro and co-lead authors Sameera Abeyrathna and Nisansala Abeyrathna, chemistry doctoral students (and siblings) in Meloni’s lab, worked together to develop the buffer formulation.
Once the biomolecules have grown a shell, they are locked in, and the lipids remain stable. While the exoskeleton is very stable, it has a fortuitous Achilles’ heel. “The shell will dissolve if it encounters something that is attracted to zinc,” Gassensmith said. “So, to release and reconstitute the liposomes, we used a zinc chelating factor called EDTA (ethylenediaminetetraacetic acid), which is a common, inexpensive food additive and medicine used to treat lead poisoning.”
In addition to the laboratory experiments, in another proof-of concept exercise, Gassensmith mailed through the U.S. Postal Service a sample of the stabilized lipid particles to his mother in Rhode Island. She shipped them back to Texas, but because the Covid-19 pandemic forced the shutdown of most UT Dallas research labs in 2020, the samples sat untouched for about two months until the graduate students returned to campus to examine them. Although the informal experiment lasted much longer than the researchers had expected, the samples survived and functioned “just fine,” Gassensmith said. “This project required two different types of expertise — my group’s expertise in membrane transport proteins and Dr. Gassensmith’s long track record working with metal-organic frameworks,” Meloni said. “Our success clearly demonstrates how such collaborative research can bring about novel and useful results.”
Source: University of Texas at Dallas
17.04.2021