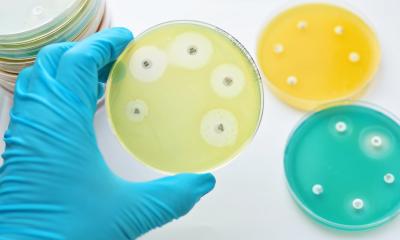
Source: Shutterstock/Nazarenko LLC
News • A new weapon against antibiotic resistance
Programming a hunter/killer toxin
When the first antibiotics were discovered in the early 20th century, the rate of death from infectious diseases fell dramatically. But the emergence of multidrug-resistant bacteria as a result of antibiotic misuse is raising fears that by 2050, these same diseases will once again become the leading cause of death worldwide.
In a bid to boost the arsenal available to tackle this threat, scientists from the Institut Pasteur, the CNRS and the Universidad Politécnica de Madrid successfully programmed a bacterial genetic structure to make it capable of specifically killing multiple antibiotic-resistant bacteria without also destroying bacteria that are beneficial to the body. Unlike other approaches under development, this novel tool is associated with a minimal rate of emergence of new resistance. The results were published in the journal Nature Biotechnology.
One of the challenges of this method is how to control the sheer power of these toxins
Didier Mazel
When an antibiotic treatment is used, the therapeutic molecules attack all the bacteria in the microbiota. This non-targeted destruction leads to dysbiosis, in other words a disruption in the balance of the bacterial ecosystem that can result in the emergence of opportunistic bacteria or resistance to the antibiotic used. The harmful impact of dysbiosis can be prevented by developing highly specific antimicrobial strategies. For example, the CRISPR-Cas9 tool can be used to target the resistance genes in pathogenic bacteria, but the rate of escape associated with the technique (when the pathogen manages to escape the various defense mechanisms employed by the infected organism) is relatively high.
In this study, a scientific team directed by Didier Mazel, Professor at the Institut Pasteur, developed an alternative strategy based on the specific expression of extremely powerful toxins delivered by conjugation. Conjugation is a process used by bacteria to exchange genes via plasmids, DNA molecules that are specific of bacterial genomes. In this novel strategy, the gene encoding the toxin is inside the plasmid. "The use of toxins from the type II toxin-antitoxin system seemed like a good idea because it appears that bacteria do not develop resistance to this arsenal", said Didier Mazel, lead author of the paper and head of the Bacterial genome plasticity unit at the Institut Pasteur. "But one of the challenges of this method is how to control the sheer power of these toxins. We did this by separating their genes into two fragments, to make sure that they would only be effective if the two parts could be recombined."

© Institut Pasteur
The scientists verified the specific nature of this toxin in Vibrio cholerae, a marine bacterium whose natural hosts are certain fish and shellfish. "We firstly wanted to activate toxin expression in Vibrio cholerae, using a promoter (a DNA region required for transcription) specifically recognized by this bacterium which expresses and activates the toxin complex," continues Didier Mazel. They then refined this "weapon" further so that the toxin would only be able to target antibiotic-resistant strains of Vibrio cholerae. This involved creating a genetic module expressing a highly specific toxin inhibitor, an antitoxin, which is no longer produced when the bacterium contains resistance genes. By combining these two procedures, they developed a genetic structure whose efficacy was verified in vivo in the complex natural communities of bacteria in the zebrafish and Artemia microbiotas. "The level of escape for this alternative strategy is very low. It can be easily adapted for the specific destruction of several other pathogens. We now need to improve the process of gene delivery by the plasmid," concludes Didier Mazel.
A patent application has been filed by the Institut Pasteur and the French National Center for Scientific Research (CNRS) for the genetic tool designed by Didier Mazel and his team and for its applications. This European patent application (EP18306780) was filed on December 20, 2018 under the name Intein mediated protein splicing system for controlled expression of proteins – Use in the expression of toxins in target cells.
Source: Institut Pasteur
16.04.2019