News • Catching flu by the stalk
One step closer to a universal influenza vaccine
Influenza viruses cause substantial health hazards and claim many lives worldwide each year. Vaccines can keep the virus in check, however, they only protect against influenza when they match the circulating strains – which vary every season. But now, a reasearch team may have found a way to generate a universal vaccine.
Led by researchers at the Icahn School of Medicine at Mount Sinai, the team uses a novel approach they’ve developed called chimeric hemagglutinin (cHA). A protein on the surface of influenza viruses, hemagglutinin, shepherds the virus into host cells. Hemagglutinin is comprised of a ‘head’ (variable) and a ‘stalk’ (varies less from strain to strain). Thus, Icahn School of Medicine researchers, led by Peter Palese, Ph.D., Professor and Chair of Microbiology and Florian Krammer, Ph.D., Professor of have focused their efforts on developing a vaccine against the stalk portion of this protein. Their study, undertaken in collaboration with PATH, an international nonprofit, the Cincinnati Children’s Hospital Medical Center, the Duke Early Phase Clinical Research Unit, and the University of Chicago, involved testing of several cHA-based vaccination regimens to see if they would induce antibodies that provide broad protection against influenza virus infections. The results were published in The Lancet Infectious Diseases.
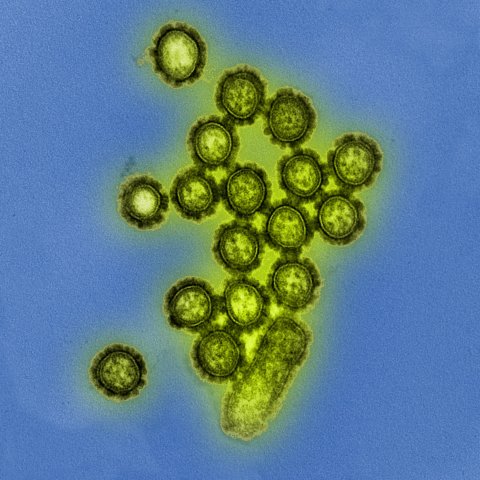
Image source: National Institutes of Health (NIH), H1N1 virus particles, marked as public domain, details at Wikimedia Commons
The diverse research team including Adolfo García-Sastre, Ph.D., Professor of Microbiology and Director of the Global Health and Emerging Pathogens Institute at Icahn; Bruce Innis, M.D., Leader, Respiratory Infections and Maternal Immunizations at PATH; and Patrick Wilson, Ph.D., Professor of Medicine at the University of Chicago, have investigated whether several potential cHA-based vaccines might induce antibodies that would target the stalk of group 1 hemagglutinin-expressing influenza viruses. An adjuvant, an ingredient that boosts the effectiveness of vaccines, was also part of the testing process.
Vaccine regimens examined included: 1) a group receiving a chimeric H8/1 hemagglutinin-based live attenuated vaccine followed by a boost with a non-adjuvanted chimeric H5/1 hemagglutinin-based inactivated vaccine (IIV), and 2) the same regimen but with the IIV having an adjuvant called AS03, and 3) a prime-boost regimen including an adjuvanted cH8/1 IIV prime followed by an adjuvanted cH5/1 IIV boost.
The researchers found that the IIV, but not the live attenuated vaccine, induced a significant antibody response after the prime, with a strong increase in anti-H1 stalk titers. All vaccine regimens induced detectable H1 stalk antibody responses after receiving boosts. “The vaccine induced a broad antibody response which was not only cross-reactive for currently circulating human influenza virus but also to avian and bat influenza virus subtypes,” said Florian Krammer. “It was surprising to find that the inactivated formulation with adjuvant induced a very strong anti-stalk response already after the prime suggesting that one vaccination might be enough to induce protection against pandemic influenza viruses yet to arise. The results indicate that we are moving towards a universal influenza virus vaccine, but these are still interim results. Additional results will be available upon completion of the study at the end of 2019.”
Source: Mount Sinai Health System
19.10.2019