News • vFFR calculation
New software facilitates angiographiy analysis
A new software to calculate the pressure drop and vFFR value (vessel Fractional Flow Reserve) in the coronary artery non-invasively was presented to interventional cardiovascular experts at EuroPCR 2018 in Paris.
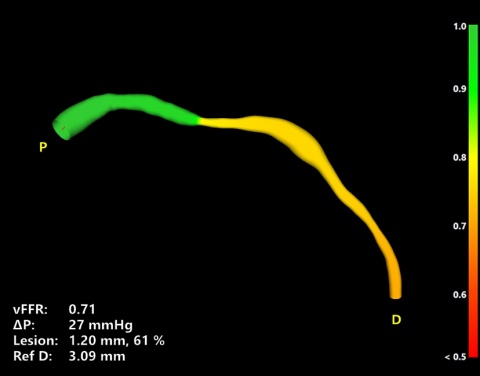
The software called CAAS vFFR (Cardiovascular Angiographic Analysis Systems for vessel Fractional Flow Reserve) was developed by Pie Medical Imaging (Esaote Group). The vFFR calculation eliminates the need for a pressure wire and hyperemic agent. FFR is an established technique used in interventional cardiology to measure pressure differences across a coronary stenosis. Based on this, cardiologist may take a decision on whether a coronary stenosis has to be treated with angioplasty or not. This examination is done during a catheterization procedure with the support of costly pressure wire and hyperemic agent.
CAAS vFFR allows clinicians to use two standard angiograms taken during a standard catheterization procedure as input to get access to coronary physiology assessment. For percutaneous coronary interventions (PCI), within one easy workflow, CAAS vFFR offers a unique combination of functional and anatomical lesion assessment (such as percentage stenosis) to support the interventional cardiologist in the clinical decision making process.
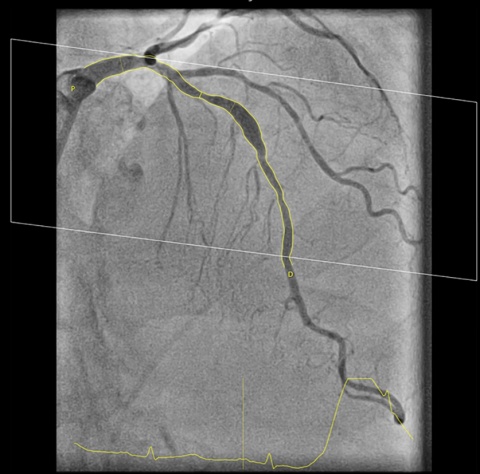
FAST, a clinical study led by Ken Masdjedi, MD and Joost Daemen MD, PhD from Erasmus Medical Center, Rotterdam shows that vFFR as calculated using CAAS vFFR has a high linear correlation to invasively measured FFR. "In the FAST study” said Joost Daemen MD, PhD, principal investigator “we demonstrated that vFFR as calculated using CAAS vFFR has a high linear correlation to invasively measured FFR and high diagnostic accuracy to detect FFR ≤ 0.80. vFFR is a promising, fast and easy to use tool to assess coronary physiology without the need for a costly pressure wire or hyperemic agent.”
“We are very proud of this technological and clinical achievement” declared René Guillaume, PMI CEO “which is the result of 30-year commitment and experience of our Company in the field of cardiovascular analysis software and of the successful collaboration with the most prestigious medical and scientific research centers”.
CAAS vFFR received USA 510(k) market clearance and is CE marked and PMDA cleared (Japan). More information on CAAS vFFR can be found at https://www.piemedicalimaging.com/product/caas-workstation/vffr/
Source: Esaote S.p.A.
25.06.2018
- angiography (117)
- cardiology (774)
- cardiovascular diseases (732)
- innovation (122)
- medical technology (1549)