News • Symbiotic cohabitation
Nerves control the body’s bacterial community
CAU research team proves, for the first time, that there is close cooperation between the nervous system and the microbial population of the body.
A central aspect of life sciences is to explore the symbiotic cohabitation of animals, plants and humans with their specific bacterial communities. Scientists refer to the full set of microorganisms living on and inside a host organism as the microbiome. Over the past years, evidence has accumulated that the composition and balance of this microbiome contributes to the organism’s health.
For instance, alterations in the composition of the bacterial community are implicated in the origin of various so-called environmental diseases. However, it is still largely unknown just how the cooperation between organism and bacteria works at the molecular level and how the microbiome and body exactly act as a functional unit.
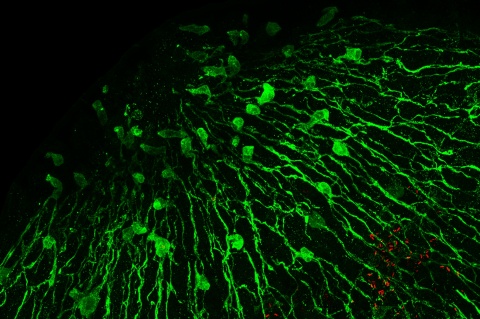
An important breakthrough in deciphering these highly complex relationships has now been achieved by a research team from Kiel University’s Zoological Institute. Using the freshwater polyp Hydra as a model organism, the Kiel-based researchers and their international colleagues investigated how the simple nervous system of these animals interacts with the microbiome. They were able to demonstrate, for the first time, that small molecules secreted by nerve cells help to regulate the composition and colonisation of specific types of beneficial bacteria along the Hydra’s body column. “Up to now, neuronal factors that influence the body’s bacterial colonisation were largely unknown. We have been able to prove that the nervous system plays an important regulatory role here,” emphasises Professor Thomas Bosch, evolutionary developmental biologist and spokesperson of the Collaborative Research Centre 1182 "Origin and Function of Metaorganisms", funded by the German Science Foundation (DFG).The scientists published their new findings in Nature Communications.
The research team, led by Bosch, use the freshwater polyp Hydra as the model organism to elucidate the fundamental principles of nervous system structure and function. Hydra represent an evolutionary ancient branch of the animal kingdom; they have a simple body plan with a nerve net of only about 3000 neurons. Applying modern experimental technology to these organisms that, despite their simplicity, still share a large molecular similarity with the nervous systems of vertebrates, enabled identification of ancient and therefore fundamental principles of nervous system structure and function.
Using this model organism, the researchers from Kiel University addressed the question of how messenger substances produced by the nervous system, known as neuropeptides, control the cooperation and communication between host and microbes. They collected cellular, molecular and genetic evidence to show that neuropeptides have antibacterial activity which affects both the composition and the spatial distribution of the colonizing microbes.
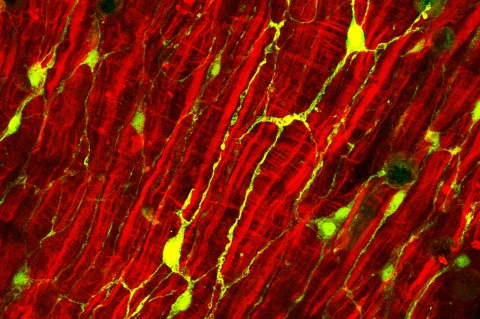
In order to reveal the connections between neuropeptides and bacterial communities, the Kiel-based researchers first concentrated on the development of the freshwater polyp’s nervous system, from the egg stage through to an adult animal. Cnidarians develop a complete nervous system within about three weeks. During this developmental time, the bacterial communities covering the animal’s surface change radically, until a stable composition of the microbiome finally forms. Under the influence of the antimicrobial effect of the neuropeptides, the concentration of so-called Gram-positive bacteria, a subgroup of bacteria, decreases sharply over a period of roughly four weeks. At the end of the maturing process, a typical composition of the microbiome prevails, particularly dominated by Gram-negative Curvibacter bacteria. Since the neuropeptides are particularly produced in certain areas of the body only, they also control the spatial localisation of the bacteria along the body column. Thus, in the head region, for example, there is a strong concentration of antimicrobial peptides, resulting in six times fewer Curvibacter bacteria than on the tentacles.
Based on these observations, the scientists concluded that throughout the course of evolution the nervous system also participated in a controlling role for the microbiome, in addition to its sensory and motor tasks. “The findings are also important in an evolutionary context. Since the ancestors of these animals have invented the nervous system, it seems that the interaction between the nervous system and the microbiome is an ancient feature of multicellular animals. Since the simple design of Hydra has great basic and translational relevance and promises to reveal new and unexpected basic features of nervous systems, further research into the interaction between body and bacteria will therefore concentrate more on the neuronal aspects,” said Bosch, to summarise the significance of the work.
Source: Christian-Albrechts-Universität zu Kiel
27.09.2017