News • DNA analysis
Magnetic biosensor array simplifies cancer detection
In standard settings, the analysis of each DNA modification requires a carefully optimised assay that runs under specific conditions. This increases cost and labour and is a severe limitation to throughput.
Now, however, researchers at Stanford University and the Technical University of Denmark have come up with a new method that will enable doctors to make a more precise diagnosis, prognosis and evaluation of the response to treatment.
Cancer arises when cells acquire errors (mutations) at certain locations in the DNA. These mutations are responsible for the characteristics of the cancer cells, and knowledge about the mutations present in a patient’s cancer is important for the diagnosis and treatment. A subset of these mutations may even be “druggable” targets, which can be directly exploited with a therapeutic intent. It has also become clear that so-called epigenetic modifications of the DNA such as methylation (adding a methyl group to the DNA) can contribute to cancer development by deactivating tumour suppressing genes. Knowing both the genetic and epigenetic profile of a patient’s cancer enables doctors to provide a more efficient diagnosis and treatment.
Genetic and epigenetic modifications can be detected using a range of molecular techniques. However, in standard settings, analysis of each DNA modification requires a carefully optimised assay that runs under specific conditions. This increases cost and labour, and is a severe limitation to throughput. Therefore, there is a need for a method that can easily and simultaneously examine a high number of genetic and epigenetic modifications.
Investigating a large number of modifications at a time
Interestingly, it was found that the measurements on the sensor array provided quantitative information on the degree of methylation, which was equivalent to that obtained by the laboratory reference method – a method called pyrosequencing
Giovanni Rizzi
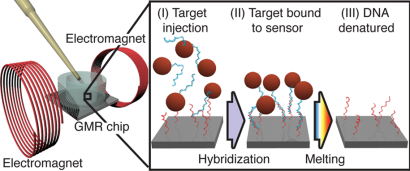
The solution relies on measurements of the DNA target using a large array of magnetic field sensors based on the Giant Magneto Resistive (GMR) effect, which is also used in read heads in magnetic hard disk drives. Each sensor in the array is used to investigate a specific sequence in the DNA. Microscopic magnetic nanoparticles are attached to the DNA to generate a signal. The biosensor array is used to investigate multiple sequences of DNA simultaneously using different probes. Varying the temperature during a single experiment is used to investigate the binding strength of the DNA and identify possible mutations. The chip method provides information comparable to the standard methods used to investigate a single or a few DNA modifications (melting curve measurements), but it does so in a scalable manner such that a large number of modifications can be investigated in a single experiment. “We demonstrated the use of a 64-sensor array for the simultaneous profiling of five mutation and four methylation sites on a series of melanoma samples,” says postdoc Giovanni Rizzi, DTU Nanotech (now at Stanford University).
The results matched those obtained in a reference laboratory. “Interestingly, it was found that the measurements on the sensor array provided quantitative information on the degree of methylation, which was equivalent to that obtained by the laboratory reference method – a method called pyrosequencing,” Giovanni Rizzi explains.
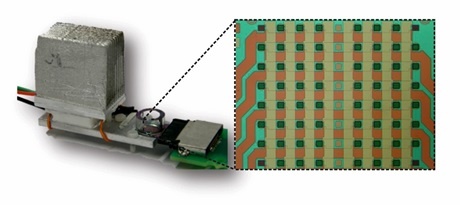
Enabling doctors to detect a broader spectrum of modifications
Giovanni Rizzi expects the method to have great potential: “The strength of the method – as I see it – is its flexibility with no need for careful optimisation of assay conditions and its scalability in the number of DNA modifications that can be investigated simultaneously.” Co-author Per Guldberg from the Danish Cancer Society agrees: “Implemented in a diagnostic setting this will enable doctors to detect and monitor a broader spectrum of genetic and epigenetic modifications in a patient’s cancer. This will enable them to make a more precise diagnosis and prediction of prognosis. Moreover, this can be done more frequently and potentially even while the patient is waiting. Thus, patients may be immediately informed of the diagnosis result within their first visit, and the doctors are ready to explain the most effective therapy to the type of cancer for which the patient is diagnosed.”
The technology is currently being further developed at Stanford University. The biosensor array will be used to analyse DNA from so-called liquid biopsies. Here, a sample of bodily fluids (as saliva, urine or blood) is used rather than a sample of cancer tissue. In such a way, the analysis could be performed in a completely non-invasive way.
Source: Technical University of Denmark
07.03.2018