News • Radiology tech
LI-RADS promotes improved liver imaging techniques and reporting
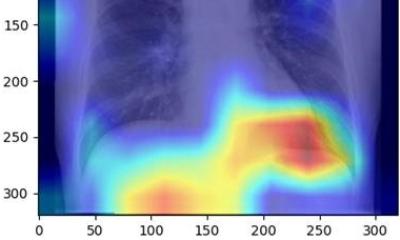
From its earliest beginnings in 2006 to its most recent update this year, the Liver Imaging Reporting and Data System (LI-RADS) is helping radiologists deliver clearer and more consistent imaging reports to hepatologists and surgeons worldwide.
"LI-RADS is the leading system for interpretation and reporting of liver imaging exams in adults with cirrhosis. It is also the first radiology system for liver imaging developed by radiologists," said Claude B. Sirlin, MD, keynote speaker and one of the founders of LI-RADS. The system was designed in response to hepatologists and surgeons who complained that terminology like "indeterminate," "worrisome" and "suspicious" was ambiguous and not actionable. According to Dr. Sirlin, professor and vice chair (translational research) of radiology at University of California, San Diego (UCSD), "LI-RADS is a blend of evidence, expert opinion and a desire for congruency with other systems of its kind. It rigorously defines and standardizes liver imaging technique, terminology, interpretation and reporting and has improved communication between radiologists and specialists diagnosing hepatocellular carcinoma (HCC) in patients with cirrhosis or those at risk for HCC.
LI-RADS was created at the UCSD around the same time as a similar system was launched at Thomas Jefferson University (TJU). In 2008, the American College of Radiology (ACR) gathered a committee of North American radiologists to use the systems created at UCSD and TJU to further develop LI-RADS. Following years of refinement, including significant work in gathering evidence and expert opinions, among other things, the current iteration is the result of several updates, each focused on tightening gaps and improving precision and simplicity.
As evidence of its growing value and influence, Dr. Sirlin noted that the Sunday series was the first RSNA session dedicated to research conducted to evaluate the accuracy and utility of LI-RADS. "Initially a North American effort by diagnostic radiologists, it is now an international consortium with over 250 contributors in 30 countries and includes not only diagnostic radiologists, but also allied specialists: interventionalists, hepatologists, surgeons and pathologists," Dr. Sirlin said.
There have been four releases of LI-RADS, including the latest version for 2017. It contains detailed processes and terminology for four areas: ultrasound (US) screening, contrast-enhanced US (CEUS), CT/MRI diagnosis and staging and CT/MRI treatment response.
LI-RADS is an adventure and a platform for international collaboration, education, mentorship, cross-fertilization, research and dissemination of knowledge
Claude B. Sirlin
Looking ahead, Dr. Sirlin said the future of LI-RADS will include more widespread adoption and additional improvements, including collection of more rigorous evidence, further refinement reflecting an international perspective, expansion beyond adults at risk for HCC, CEUS treatment response evaluation assisted interpretation and reporting and deep learning to augment and facilitate the use of LI-RADS. Given its expansion and the improvements it has delivered to the area of HCC diagnosis, Dr. Sirlin said he anticipates LI-RADS will enable the development of international registries to accelerate progress across the entire field of liver imaging while ensuring that radiologists remain at the forefront of the effort. "LI-RADS is an adventure and a platform for international collaboration, education, mentorship, cross-fertilization, research and dissemination of knowledge," he said.
Copyright: RSNA Daily Bulletin/Lynn Antonopoulos
28.11.2017