Image source: NYCU; adapted from: Chiu PH, Lai KC, Wang HL et al., Cell Reports Medicine 2025 (CC BY 4.0)
News • Focus on tumor STAT1 acetylation
How cancer cells learn under pressure to evade immunotherapy
The findings show that prolonged targeted therapy in patients with head and neck cancer can heighten tumor alertness, reducing the effectiveness of subsequent immunotherapy.
Immunotherapy has been hailed as a breakthrough in cancer treatment, earning global recognition with the Nobel Prize in Physiology or Medicine. But new research from National Yang Ming Chiao Tung University (NYCU) reveals a sobering reality: under sustained treatment pressure, cancer cells do not simply weaken — they adapt, learn, and fight back.
A research team from the Institute of Clinical Medicine at NYCU has found that cancer cells exposed to long-term targeted therapy can develop heightened “stress resilience,” enabling them to evade subsequent immunotherapy. The findings help explain why many patients with head and neck cancer fail to achieve the expected outcomes when immunotherapy is administered after prolonged targeted treatment. The study was published in Cell Reports Medicine.
By understanding how tumors adapt under therapeutic stress, we may be able to use biomarkers to guide treatment order and combination strategies — ultimately improving the success rate of immunotherapy
Muh-Hwa Yang
By closely analyzing tumor behavior under extended targeted therapy, the researchers uncovered a critical mechanism behind immunotherapy resistance. Rather than remaining vulnerable, cancer cells respond to prolonged drug pressure by rapidly remodeling the tumor microenvironment. In some cases, they actively shut down signaling pathways that would normally activate immune cells, effectively rendering immunotherapy ineffective.
At the core of this adaptive response is the inflammatory cytokine tumor necrosis factor alpha (TNF-α). When targeted drugs chronically suppress tumors, they begin to secrete large amounts of TNF-α, which interferes with STAT1 — a key regulator that activates interferon-driven anti-tumor genes. This disruption leads to a phenomenon known as “interferon-gamma fatigue,” in which immune cells gradually lose their ability to recognize and attack cancer cells.
In a separate but complementary study published in Advanced Science, the NYCU team collaborated with Academia Sinica Academician Mien-Chie Hung to uncover another immune-evasion strategy used by cancer cells. The researchers identified RNase1, an enzyme secreted by tumors, that directly suppresses the activity of T cells and other immune cells.

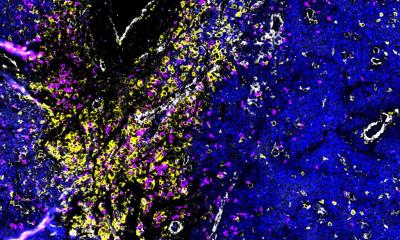
Image source: NYCU
This mechanism was observed across multiple cancer types — including breast cancer, liver cancer, and head and neck cancer — suggesting that RNase1 is a cross-cancer immune escape factor with broad clinical significance.
Taken together, the two studies paint a clear picture of cancer as a highly adaptive system. Under therapeutic pressure, cancer cells do not merely passively resist treatment. Instead, they actively reprogram immune signaling pathways, secrete proteins that weaken immune attacks, and learn how to survive in increasingly hostile environments. “Cancer cells grow under pressure,” the researchers noted, demonstrating an evolutionary resilience that challenges current treatment strategies.
Despite the sobering findings, the research also offers a path forward. Understanding how tumors adapt under treatment pressure provides clinicians with valuable guidance for optimizing treatment sequencing, combination therapies, and biomarker-driven decision-making.
“Immunotherapy represents a major milestone in cancer treatment, but overcoming resistance remains one of the greatest clinical challenges,” said Professor Muh-Hwa Yang of NYCU, a senior author of the studies. “By understanding how tumors adapt under therapeutic stress, we may be able to use biomarkers to guide treatment order and combination strategies — ultimately improving the success rate of immunotherapy.”
The findings underscore the importance of viewing cancer treatment not as a single intervention, but as a dynamic process — one in which timing, sequencing, and biological context may determine success or failure.
Source: National Yang Ming Chiao Tung University; edited by Chance Lai
14.01.2026