Herceptin destroys breast cancer stem cells
The HER2 gene causes a very aggressive kind of breast cancer. It influences the number of cancer stem cells and therefore as well the spreading of metastases. The University of Michigan Comprehensive Cancer Center now made the discovery that the drug Herceptin can drastically reduce the amount of the stem cell population in HER2 positive-breast cancer.
A gene that is overexpressed in 20 percent of breast cancer increases the number of cancer stem cells, the cells that fuel a tumor's growth and spread, according to a new study from the University of Michigan Comprehensive Cancer Center.
The gene, HER2, causes cancer stem cells to multiply and spread, explaining why HER2 has been linked to a more aggressive type of breast cancer and to metastatic disease, in which the cancer has spread beyond the breast, the researchers say.
Further, the drug Herceptin, which is used to treat HER2-positive breast cancer, was found to target and destroy the cancer stem cells. Results of the study appear online in the journal Oncogene.
"This work suggests that the reason drugs that target HER2, such as Herceptin and Lapatanib, are so effective in breast cancer is that they target the cancer stem cell population. This finding provides further evidence for the cancer stem cell hypothesis," says study author Max S. Wicha, Distinguished Professor of Oncology and director of the U-M Comprehensive Cancer Center.
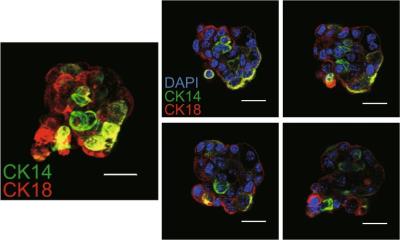
The cancer stem cell hypothesis says that tumors originate in a small number of cells, called cancer stem cells, and that these cells are responsible for fueling a tumor’s growth. These cells represent fewer than 5 percent of the cells in a tumor. Wicha’s lab was part of the team that first identified stem cells in human breast cancer in 2003.
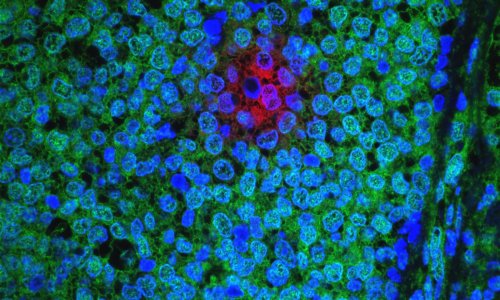
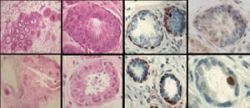
In the current study, researchers found that breast cancer cells overexpressing the HER2 gene had four to five times more cancer stem cells, compared to HER2-negative cancers. In addition, the HER2-positive cells caused the cancer stem cells to invade surrounding tissue, suggesting that HER2 is driving the invasiveness and spread of cancer.
The researchers then looked at the drug Herceptin, which is used to treat HER2-positive breast cancer. They found Herceptin reduced the number of cancer stem cells in the HER2-positive breast cancer cell lines by 80 percent, dropping it to the same levels seen in HER2-negative cell lines.
When HER2 was not overexpressed in the cell cultures, the researchers found, the cancer stem cell population did not increase. Nor did Herceptin have any effect on the HER2-negative cells, which is consistent with how Herceptin is used in the clinic.
"We are now studying what pathways are activated by HER2 overexpression. Our hope is that we could develop inhibitors of these pathways that might be effective in targeting cancer stem cells in women whose tumors do not overexpress HER2 or those who are resistant to Herceptin," says study author Hasan Korkaya, Ph.D., a U-M research fellow in internal medicine.
Source: University of Michigan
16.07.2008