News • "Gene scissors" vs. sickle cell disease
FDA approves first CRISPR/Cas therapy
The U.S. Food and Drug Administration (FDA) approved two milestone treatments, Casgevy and Lyfgenia, representing the first cell-based gene therapies for the treatment of sickle cell disease (SCD) in patients 12 years and older.
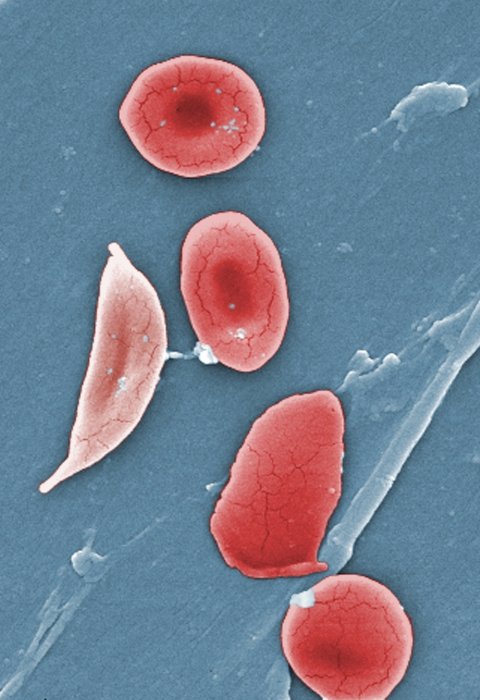
Image source: CDC/ Sickle Cell Foundation of Georgia: Jackie George, Beverly Sinclair
Additionally, one of these therapies, Casgevy, is the first FDA-approved treatment to utilize a type of novel genome editing technology, signaling an innovative advancement in the field of gene therapy.
Sickle cell disease is a group of inherited blood disorders affecting approximately 100,000 people in the U.S. It is most common in African Americans and, while less prevalent, also affects Hispanic Americans. The primary problem in sickle cell disease is a mutation in hemoglobin, a protein found in red blood cells that delivers oxygen to the body’s tissues. This mutation causes red blood cells to develop a crescent or “sickle” shape. These sickled red blood cells restrict the flow in blood vessels and limit oxygen delivery to the body’s tissues, leading to severe pain and organ damage called vaso-occlusive events (VOEs) or vaso-occlusive crises (VOCs). The recurrence of these events or crises can lead to life-threatening disabilities and/or early death.
“Sickle cell disease is a rare, debilitating and life-threatening blood disorder with significant unmet need, and we are excited to advance the field especially for individuals whose lives have been severely disrupted by the disease by approving two cell-based gene therapies today,” said Nicole Verdun, M.D., director of the Office of Therapeutic Products within the FDA’s Center for Biologics Evaluation and Research. “Gene therapy holds the promise of delivering more targeted and effective treatments, especially for individuals with rare diseases where the current treatment options are limited.”
Casgevy, a cell-based gene therapy, is approved for the treatment of sickle cell disease in patients 12 years of age and older with recurrent vaso-occlusive crises. Casgevy is the first FDA-approved therapy utilizing CRISPR/Cas9, a type of genome editing technology. Patients’ hematopoietic (blood) stem cells are modified by genome editing using CRISPR/Cas9 technology. CRISPR/Cas9 can be directed to cut DNA in targeted areas, enabling the ability to accurately edit (remove, add, or replace) DNA where it was cut. The modified blood stem cells are transplanted back into the patient where they engraft (attach and multiply) within the bone marrow and increase the production of fetal hemoglobin (HbF), a type of hemoglobin that facilitates oxygen delivery. In patients with sickle cell disease, increased levels of HbF prevent the sickling of red blood cells.
Recommended article

News • For the development of CRISPR/Cas9
Nobel Prize in Chemistry goes to Emmanuelle Charpentier and Jennifer A. Doudna
The Royal Swedish Academy of Sciences has decided to award the Nobel Prize in Chemistry 2020 to Emmanuelle Charpentier from the Max Planck Unit for the Science of Pathogens, Berlin, Germany, and Jennifer A. Doudna from the University of California, Berkeley, USA, “for the development of a method for genome editing”, more commonly known as the 'gene scissors' CRISPR/Cas9.
Lyfgenia is a cell-based gene therapy. Lyfgenia uses a lentiviral vector (gene delivery vehicle) for genetic modification and is approved for the treatment of patients 12 years of age and older with sickle cell disease and a history of vaso-occlusive events. With Lyfgenia, the patient’s blood stem cells are genetically modified to produce HbAT87Q, a gene-therapy derived hemoglobin that functions similarly to hemoglobin A, which is the normal adult hemoglobin produced in persons not affected by sickle cell disease. Red blood cells containing HbAT87Q have a lower risk of sickling and occluding blood flow. These modified stem cells are then delivered to the patient.
These approvals represent an important medical advance with the use of innovative cell-based gene therapies to target potentially devastating diseases and improve public health
Peter Marks
Both products are made from the patients’ own blood stem cells, which are modified, and are given back as a one-time, single-dose infusion as part of a hematopoietic (blood) stem cell transplant. Prior to treatment, a patients’ own stem cells are collected, and then the patient must undergo myeloablative conditioning (high-dose chemotherapy), a process that removes cells from the bone marrow so they can be replaced with the modified cells in Casgevy and Lyfgenia. Patients who received Casgevy or Lyfgenia will be followed in a long-term study to evaluate each product’s safety and effectiveness.
“These approvals represent an important medical advance with the use of innovative cell-based gene therapies to target potentially devastating diseases and improve public health,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research. “Today’s actions follow rigorous evaluations of the scientific and clinical data needed to support approval, reflecting the FDA’s commitment to facilitating development of safe and effective treatments for conditions with severe impacts on human health.”
The safety and effectiveness of Casgevy were evaluated in an ongoing single-arm, multi-center trial in adult and adolescent patients with SCD. Patients had a history of at least two protocol-defined severe VOCs during each of the two years prior to screening. The primary efficacy outcome was freedom from severe VOC episodes for at least 12 consecutive months during the 24-month follow-up period. A total of 44 patients were treated with Casgevy. Of the 31 patients with sufficient follow-up time to be evaluable, 29 (93.5%) achieved this outcome. All treated patients achieved successful engraftment with no patients experiencing graft failure or graft rejection.
The most common side effects were low levels of platelets and white blood cells, mouth sores, nausea, musculoskeletal pain, abdominal pain, vomiting, febrile neutropenia (fever and low white blood cell count), headache and itching.
The safety and effectiveness of Lyfgenia is based on the analysis of data from a single-arm, 24-month multicenter study in patients with sickle cell disease and history of VOEs between the ages of 12- and 50- years old. Effectiveness was evaluated based on complete resolution of VOEs (VOE-CR) between 6 and 18 months after infusion with Lyfgenia. Twenty-eight (88%) of 32 patients achieved VOE-CR during this time period.
The most common side effects included stomatitis (mouth sores of the lips, mouth, and throat), low levels of platelets, white blood cells, and red blood cells, and febrile neutropenia (fever and low white blood cell count), consistent with chemotherapy and underlying disease.
Hematologic malignancy (blood cancer) has occurred in patients treated with Lyfgenia. A black box warning is included in the label for Lyfgenia with information regarding this risk. Patients receiving this product should have lifelong monitoring for these malignancies.
Both the Casgevy and Lyfgenia applications received Priority Review, Orphan Drug, Fast Track and Regenerative Medicine Advanced Therapy designations.
The FDA granted approval of Casgevy to Vertex Pharmaceuticals Inc. and approval of Lyfgenia to Bluebird Bio Inc.
Source: U.S. Food and Drug Administration
12.12.2023