Surfing DNA
Enzyme catches a ride to fight infection
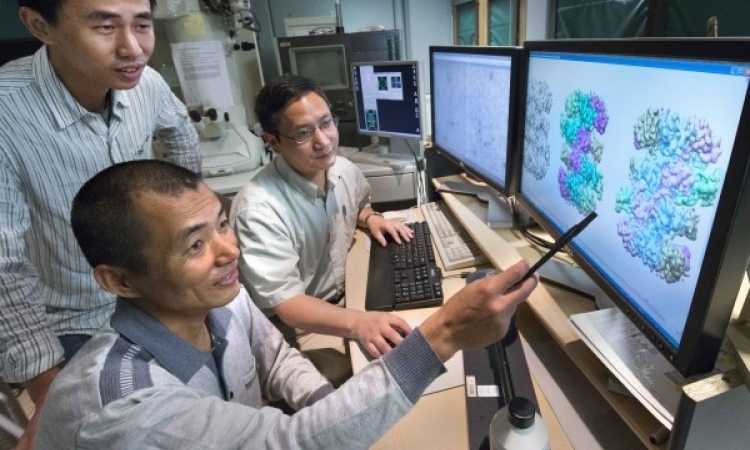
Scientists have shown for the first time that an enzyme crucial to keeping our immune system healthy “surfs” along the strands of DNA inside our cells. The researchers used extremely powerful microscopy to watch how the enzyme AID (activation-induced deoxycytidine deaminase) moves around and interacts with other molecules.

They were able to see that it catches a free ride on top of another enzyme, which has its own motor. “Before now, no one has shown that an enzyme can ride on top of a motor-enzyme,” says David Rueda who leads the Single Molecule Imaging group at the MRC Clinical Sciences Centre at Imperial College, and who led the team whose research is published today in Nature Communications.
AID helps our immune system to recognise and respond to pathogens such as bacteria and viruses. Each strain of pathogen has a unique pattern of molecules on its surface. These molecules come in all shapes and sizes, so that the immune system can distinguish between different pathogens.
Small proteins called antibodies bind to these molecules. Once bound, the antibodies act as a guide for the immune system by flagging up pathogens for destruction. But each antibody binds only to one particular shape. To respond to new pathogens, our bodies must constantly make new antibodies with new shapes. This is where AID comes in.
When immune cells produce antibodies, they must first make a copy of the gene, or section of DNA, that contains information about how to make an antibody. AID makes subtle changes, or mutations, to this section of DNA in random places. Some of these random mutations will result in the production of antibodies that “fit” the shapes of pathogens.
To mutate the DNA, scientists knew that AID must first move into position on the strand but they didn’t know how this happened. Rueda’s team is the first to observe its movements in real time.
The new findings show that AID is drawn towards the DNA strand when antibody production begins. In this initial step the DNA is copied by an enzyme, called a polymerase, which draws power from its motor to move along the strand.
As the polymerase moves, it makes a bubble and AID surfs this bubble much like a surfer rides a wave. They move together, and during this time AID makes random mutations in the DNA. Eventually, AID makes polymerase stall and, like a surfer on a wave that has reached the shore, AID and the polymerase come to a halt and fall off or move away from the strand.
“What’s interesting is that the polymerase has a motor that allows it to move in one direction but AID doesn’t, so AID takes advantage of the polymerase motor to follow it,” says Rueda. “We don’t know all the molecular details of the mechanism yet, but we’ve shown that AID follows the polymerase, and that this is how it goes from one side of the DNA to another.”
The microscopy technique used by the team is called single-molecule fluorescence resonance energy transfer, or smFRET. This allowed them to see exactly what the AID molecule was doing at any given moment. Although smFRET was developed almost two decades ago, the team is the first to use it to explore how AID manoeuvres itself into position on a strand of DNA.
On occasion, AID mutates the DNA in the wrong place or at the wrong time, and this can contribute to the development of a range of cancers, including breast, colon and lung cancer. According to Rueda, if scientists can find out more about AID and what happens when it goes wrong, they may be able to develop approaches to control its action, and this may one day help to develop new treatments for these types of cancer.
Source: MRC Clinical Sciences Centre
04.01.2016