Image source: Carrasco Yagüe M, Zhang X, Volpatti M, et al., Science Advances 2025 (CC BY-NC 4.0)
News • Electrical impedance spectroscopy
EIS shows promise to advance cancer cell monitoring
A new study unveils a method to non-invasively monitor cell spatiotemporal dynamics involved in cancer progression in a real-time and label-free manner, which can provide new insights for cancer diagnosis and treatment.
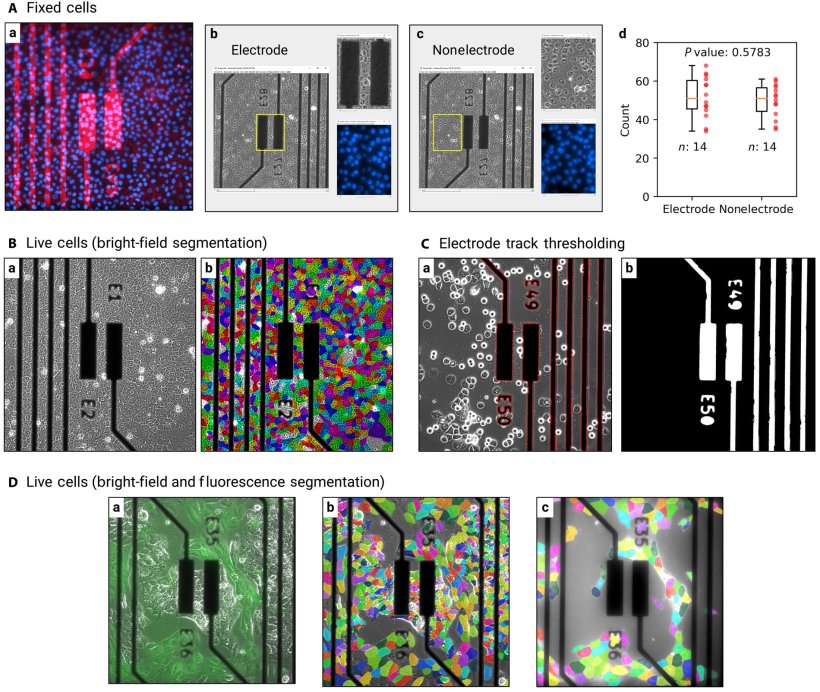
The new methodology combines the use of micro-electrode arrays, electrical impedance spectroscopy (EIS) that measures the characteristics of tissue surrounding the sensor, and predictive algorithms. The use of predictive algorithms allows for faster prediction, better resilience to noise, and recognition of complex data patterns when compared to traditional EIS analysis approaches. This new methodology is believed to be the first-ever use of EIS to enable quantitative real-time monitoring of cell spatiotemporal dynamics, or cell changes over time.
This technology has the potential to obviate the need for microscopy imaging in cancer cell monitoring in various settings and significantly advance our understanding of cancer cell behavior and interactions
Abdul Barakat
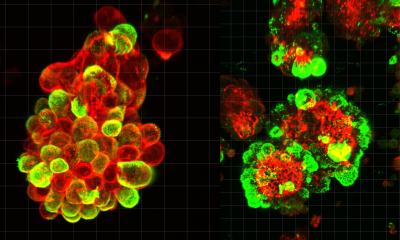
The research was a multi-year endeavor led by Sensome and École Polytechnique, in collaboration with the Center for Nanoscience and Nanotechnology (C2N). In the study, the team at École Polytechnique’s Hydrodynamics Laboratory (LadHyX*) leveraged Sensome’s technology and exposed it to normal and cancerous breast epithelial cells, where it was able to accurately predict the spatiotemporal evolution of cell density, cell substrate coverage, mean cell diameter, and cell type in agreement with microscopy findings. It also enabled real-time tracking of spatial heterogeneities in breast cancer cell growth and the competition between normal and cancerous cells based on the EIS measurements alone.
“This technology has the potential to obviate the need for microscopy imaging in cancer cell monitoring in various settings and significantly advance our understanding of cancer cell behavior and interactions,” said co-author Abdul Barakat, CNRS Director of Research and Professor at École Polytechnique. “Assessing how cells organize in space and time is essential to elucidating cancer progression. Live-cell fluorescence microscopy is the predominant method for tracking these dynamics today but is often limited by cytotoxicity induced by the fluorescent dyes, by cellular photo-damage during extended periods of microscopic imaging, and by restrictions in optical access in the case of opaque clinical samples. This methodology using Sensome’s technology demonstrates a non-invasive, label-free method enabling long-term monitoring of cancer-related cellular spatiotemporal dynamics with minimal disruption of natural cellular processes.”
“This study shows that the proprietary signal processing and machine learning algorithms involved in our technology can empower a method to successfully monitor cancer cell differentiation and evolution over time,” said Franz Bozsak, CEO and co-founder of Sensome. “This breakthrough is the first step in exploring the use of our tissue-sensing technology in monitoring cancer-related phenomena, such as tumor growth. It complements the work we are currently doing in lung cancer—where in-situ cancer detection is crucial—which is one of several applications where we are applying our mastery of electrical impedence spectroscopy to novel uses in medicine. We are actively seeking industrial partners to realize innovative medical devices centered on our technology.”
The Sensome tumor sensing technology is an investigational device and is not approved for commercial use in the U.S or any other jurisdiction.
*LadHyX: a joint research unit CNRS, École Polytechnique - Institut Polytechnique de Paris
Source: École Polytechnique - Institut Polytechnique de Paris
22.07.2025