News • Implant and app
New device monitors bladder fullness
Northwestern University researchers have developed a new soft, flexible, battery-free implant that attaches to the bladder wall to sense filling.

Image credit: Northwestern University
Then, it wirelessly — and simultaneously — transmits data to a smartphone app, so users can monitor their bladder fullness in real time. The study is published in the Proceedings of the National Academy of Sciences (PNAS). It marks the first example of a bioelectronic sensor that enables continuous monitoring of bladder function for a prolonged period.
While this new device is unnecessary for the average person, it could be a game-changer for people with paralysis, spina bifida, bladder cancer or end-stage bladder disease — where bladder function is often compromised, and bladder reconstruction surgery may be required. The sensor system also can enable clinicians to monitor their patients remotely and continuously to make more informed — and faster — treatment decisions. “If bladder nerves are damaged from surgery or from a disease such as spina bifida, then a patient often loses sensation and is unaware that their bladder is full,” said Northwestern’s Guillermo A. Ameer, who co-led the work. “To empty the bladder, they often have to use catheters, which are uncomfortable and can lead to painful infections. We want to eliminate the use of catheters and bypass current bladder function monitoring procedures, which are highly invasive, very unpleasant and must be done in a hospital or clinical setting.”
An expert in regenerative engineering, Ameer is the Daniel Hale Williams Professor of Biomedical Engineering at Northwestern’s McCormick School of Engineering and professor of surgery at Northwestern University Feinberg School of Medicine. He also directs the Center for Advanced Regenerative Engineering and the predoctoral Regenerative Engineering Training Program, funded by the National Institutes of Health. Ameer co-led the study with Northwestern’s John A. Rogers and Arun Sharma. A bioelectronics pioneer, Rogers is the Louis Simpson and Kimberly Querrey Professor of Materials Science and Engineering, Biomedical Engineering and Neurological Surgery at McCormick and Feinberg. He also directs the Querrey Simpson Institute for Bioelectronics. Sharma is a research associate professor of urology at Feinberg and of biomedical engineering at McCormick. He also is director of pediatric urological regenerative medicine at Stanley Manne Children’s Research Institute at Ann & Robert H. Lurie Children’s Hospital of Chicago.
Due to problems with nerves, brain or spinal cord, millions of Americans suffer from dysfunctional bladders. These issues can arise from congenital defects such as spina bifida — where a person is born with a damaged spine — or traumatic injuries sustained at any point in life. When left untreated, severe bladder dysfunction can cause routine infections and urination issues, eventually leading to kidney damage, which affects the entire body. Enabling physicians to remotely monitor their patients could enable faster interventions.
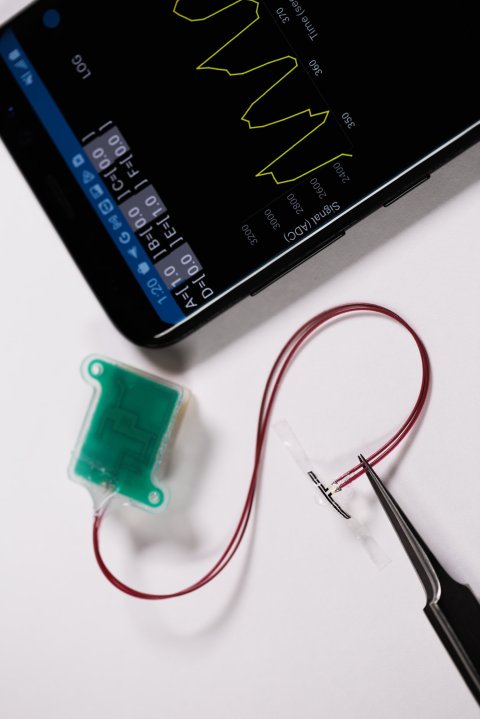
Image credit: Northwestern University
To monitor the bladder, the new device comprises multiple sensors, which work together to measure one simple parameter: strain. As the bladder fills, it expands. The fuller the bladder becomes, the more it stretches. This stretching pulls on the elastic-like device to signal strain. Likewise, when the bladder empties, it contracts, which then relieves strain. As the sensors detect various levels of strain, the device uses embedded Bluetooth technology to transmit this information to a smartphone or tablet. “The key advance here is in the development of super soft, ultrathin, stretchable strain gauges that can gently wrap the outside surface of the bladder, without imposing any mechanical constraints on the natural filling and voiding behaviors,” Rogers said.
In small animal studies, the system successfully delivered real-time measurements of bladder filling and emptying for 30 days. Then, in a study using non-human primates, the system successfully delivered information for eight weeks. The researchers also demonstrated that the sensors are sensitive enough to detect strain from very low volumes of urine. “This work is the first of its kind that is scaled for human use,” Ameer said. “We demonstrated the potential long-term function of the technology. Depending on the use case, we can design the technology to reside permanently inside the body or to harmlessly dissolve after the patient has made a full recovery.” Although the new technology is useful on its own, Ameer envisions it as one component of a fully integrated system for bladder function restoration.
Just last month, Ameer and Sharma introduced a biodegradable synthetic, flexible “bladder patch,” which was published in PNAS Nexus. Seeded with the patient’s own stem cells, the citrate-based “patch” — referred to as a pro-regenerative scaffold (PRS) — enables the surgeon to reconstruct or rebuild the bladder without the need to harvest intestinal tissue, the current clinical standard for this surgery. The “patch,” which expands and contracts with the native bladder tissue, supports the migration and growth of bladder cells. Then it slowly dissolves, leaving behind new bladder tissue. The researchers demonstrated that the new tissue remained functional throughout the two-year study.
In addition to monitoring the filling, the app will be able to send warnings to the patient and then direct them to locations for the nearest restrooms. Also, one day, patients will be able to trigger urination, on demand, through their smartphone
Guillermo A. Ameer
“We are working to integrate our bladder regeneration technology with this novel wireless bladder monitoring technology to restore bladder function and monitor the recovery process after surgery,” Ameer said. “This work brings us closer to the reality of smart regenerative systems, which are implantable pro-regenerative devices capable of probing their microenvironment, wirelessly reporting those findings outside the body (to the patient, caregiver or manufacturer) and enabling on-demand or programmed responses to change course and improve device performance or safety.”
“This technology represents a significant advancement, as there are currently no other tissue engineering-based approaches available to these patients,” Sharma said. “I am confident this will help improve the quality of life for many patients who will now be able to avoid the use of intestinal tissues and its myriad complications.”
Ameer continues to work with Rogers and Sharma to build new functionalities into the system. They currently are exploring ways that the implant could stimulate the bladder to induce urination on demand. “In addition to monitoring the filling, the app will be able to send warnings to the patient and then direct them to locations for the nearest restrooms,” Ameer said. “Also, one day, patients will be able to trigger urination, on demand, through their smartphone.”
Source: Northwestern University
26.03.2024