Image source: Rizzotto D et al., Science Advances 2024 (CC BY 4.0)
News • Research on "double strike" mechanism
Induction of defective cell division could improve blood cancer therapy
Unseen and ongoing, thousands of times every second: to keep a complex organism like humans alive, an immense number of new cells must be continuously produced.
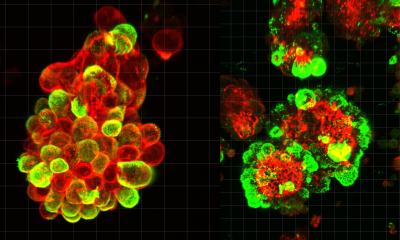
Up close, each of these cell divisions is nothing short of a miracle. Within just a few hours, not only must the entire genome – billions of “letters” long – be replicated, but most other cellular structures must be doubled so that, in the end, two complete daughter cells can emerge. Just before division, two complex protein structures, known as centrosomes, emerge, forming two opposing poles in the mother cell. These centrosomes grow long protein filaments, the spindle apparatus, that stretch toward the duplicated genetic material, latch onto it, and pull one copy of each chromosome to the opposing centrosome, evenly distributing the genetic material to the emerging daughter cells.
However, if this process fails, results can be catastrophic. The resulting cells, locked together like Siamese twins, would have twice as many chromosomes and centrosomes, making them unfit and prone to malignancy. Many cancer cells, for example, exhibit abnormal chromosome numbers and extra centrosome. Typically, before such defective cells can survive and grow, they arrest their cell cycle, or trigger their own destruction by an unknown mechanism.

Image credit: Wolfgang Däuble / CeMM
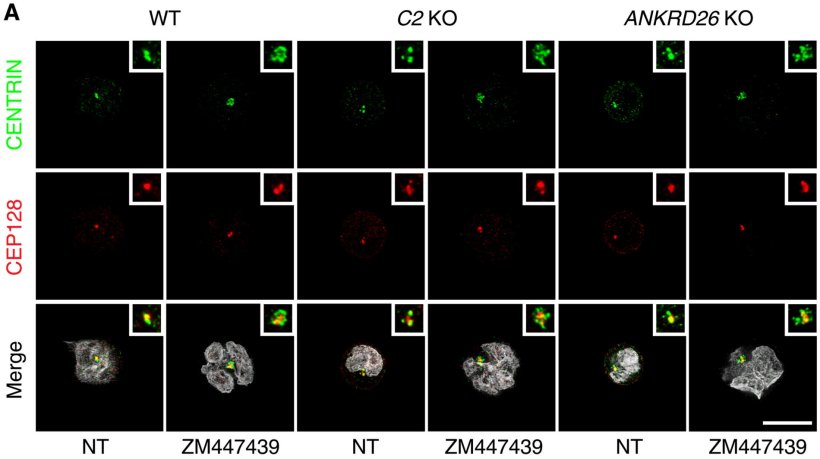
Researchers led by Andreas Villunger (Adjunct Principal Investigator at CeMM in Vienna and Professor at the Medical University of Innsbruck) and Luca Fava (Associate Professor at the University of Trento, Italy) have now clarified how this programmed cell death – known as apoptosis – is set off during faulty cell division. In their study, published in Science Advances, they found that the presence of multiple centrosomes in a cell, a hallmark of disrupted division, activates a large protein complex called the PIDDosome.
The PIDDosome, in turn, activates the enzyme caspase-2, triggering two lethal pathways. First, it activates the protein BID, which directly destroys the mitochondria – the cell's powerhouses – leading to cell death. Simultaneously, caspase-2 activates the well-known tumor suppressor p53, which initiates additional signaling pathways that also result in cell death. This “double strike” ensures that cells with multiple centrosomes are eliminated, even under conditions when either BID or p53 are lacking or inhibited.

Image credit: University of Trento
The researchers’ findings not only shed new light on these fundamental molecular mechanisms but also suggest potential applications in blood cancer treatment. Tumor cells are notorious for their rapid and uncontrolled division, and many cancer therapies aim to disrupt this process. Often, this leads to the formation and accumulation of multiple centrosomes in cancer cells, and the deadly effect of the PIDDosome could be harnessed to improve the efficacy of such treatments.
“By analyzing the BID and caspase-2 activity in cancer cells, we could potentially identify patients who are most likely to respond to drugs that interfere with cell division,” explains Andreas Villunger, highlighting the potential clinical application of their research. “Translating laboratory research into clinical practice is a lengthy and complex process. However, gaining deeper insights into the mechanisms of already approved drugs is essential to making therapies both more effective and less invasive”, adds Luca Fava, who believes that the research could help to layout the use of new combinations of existing drugs.
Source: CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences
31.10.2024