Article • A potentially devastating impact
Covid-19 alters antibiotic use
The long-term impact of the coronavirus pandemic on antimicrobial resistance (AMR) remains difficult to predict. Infectious diseases consultant Professor Alison Holmes said Covid-19 has impacted on antibiotic use in hospitals and across the wider community, but more research is needed to fully assess the extent and implications.
Report: Mark Nicholls

Holmes discussed the subject in detail in her presentation ‘Healthcare associated infection, antibiotic use, drug resistance and Covid-19’, to the 73rd annual congress of the German Society for Hygiene and Microbiology, with a focus on bacterial infections, antibiotic use, hospital-onset Covid infection and how that should be tackled, and healthcare associated infections (HCAI) in individuals with and without Covid-19.
‘Bacterial infection and Covid-19 were a major concern, particularly at the beginning of the pandemic in terms of driving antibiotic use and potential AMR,’ said Holmes, Professor of Infectious Diseases and Director of the NIHR Health Protection Research Unit in Healthcare Associated Infections and AMR, and the Centre for Antimicrobial Optimisation (CAMO) at Imperial College London.
Antibiotic prescribing
Emphasizing the importance of looking at the effect, directly and indirectly, of other clinical activity related to infections on Covid and non-Covid populations, she said: ‘There were many consequences that could impact on infection that could fuel AMR, or indeed reduce it significantly, and it is important that we consider this across multiple levels.’
Further considerations included the potential for bacterial infection risk on new therapies, particularly immunological; the pressures on different healthcare systems, and patient backlogs. Holmes, who has contributed to the national Covid-19 response in the UK, noted that early in the pandemic, experts were keen to conduct a rapid review in terms of bacterial and fungal infections, but particularly how it could support antibiotic prescribing.
Low levels of coinfection in Covid-19 (8%), but high rates of antimicrobial use (70%), were suggested in initial research, but there were research limitations, which often did not differentiate whether patient populations were in critical care or not; there was limited microbiology, risk factors such as whether patients were being ventilated not being reported; and complications of excessive prescribing were not identified. She said the evidence at the time supported use of antibiotics only in suspected or confirmed serious infections and with regular review, short duration and the need to maintain strong antimicrobial stewardship programmes, though more recent evidence has ‘expanded the level of understanding of coinfection’.
Critical care has been highlighted as the main focus for coinfection, with organisms linked predominantly to local epidemiology. But while the majority of coinfections were hospital-acquired pneumonia or ventilator-associated pneumonia, there was a need for these risk factors to be collected in a standardised way to ‘understand risk of Covid in terms of coinfection’.
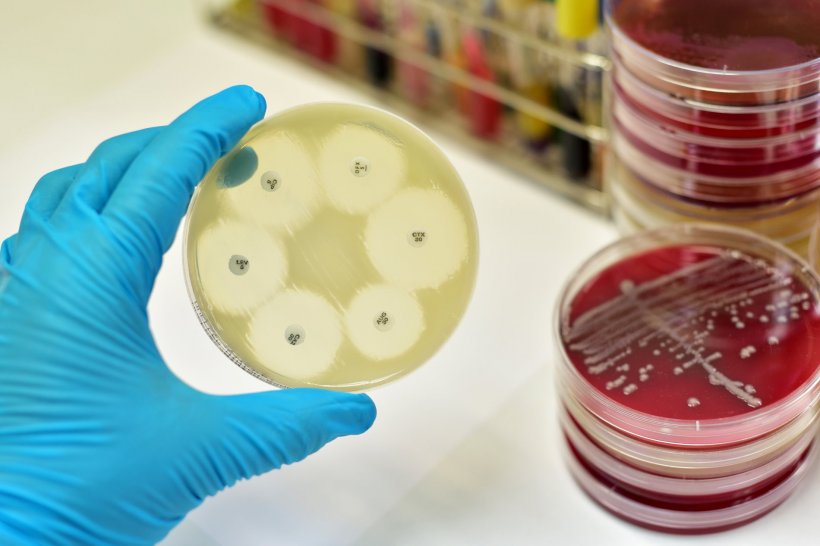
Image source: Shutterstock/Jarun Ontakrai
Impact on non-Covid patients
More recent study findings, however, were not dissimilar to the early research, with 8.6% of bacterial infections seen versus 74.6% of patients on antibiotics. But she added: ‘We are not doing well enough in terms of understanding what is going on in low resource settings and we need to do much better around that to understand the potential devasting impact this is having on our non-Covid patients.’
Holmes also discussed the impact of Covid on bacteremias, notably a major drop in across the two waves of Covid and particularly in e.coli bacteremia, though this was tempered by the change in the patient mix. In the USA, she noted, there was a rise in hospital acquired infections in 2020 compared to 2019, after years of reductions, mainly catheter associated urinary tract infections, ventilator associated events, and MRSA, but notably no increase in surgical site infections (with surgery being cancelled), and no increase in C.diff rates.
Community prescribing
With concerns about AMR in acute care, the implications of a fall in antibiotic use in the community – traditionally an area of higher usage – remain uncertain. ‘That’s a major concern because we don’t know if people could access treatment appropriately, or if patients with infections are going untreated. There is a need for better evidence on this,’ Holmes said, and emphasised the need for ‘strong, robust surveillance’ of hospital-onset Covid for future pandemic preparedness, and recovery of healthcare resilience and safety. Health systems need to understand that non-Covid patients can be well looked after and health systems be run effectively and safely with the appropriate level of capacity in terms of beds and staffing, she underlined.
Local epidemiology
By recognising that the AMR pandemic is happening already, we need to be able to understand and make the most of the information, so that we can learn from the Covid pandemic
Alison Holmes
While bacterial infection in Covid patients is relatively low, with the majority in acute care due to HCAIs, she said understanding the local epidemiology is critical and that a strong foundation of effective prevention and control practice is vital.
Antibiotic stewardship is also crucial given the high rates of prescribing, and there is a need for a better framework for prospective reporting to inform clinicians in providing safe healthcare for HCAIs associated with Covid, for hospital-onset Covid infections, and for antibiotic use within the Covid pandemic. ‘By recognising that the AMR pandemic is happening already, we need to be able to understand and make the most of the information, so that we can learn from the Covid pandemic,’ the professor said. ‘The long-term impact of the pandemic on AMR is still difficult to predict and it’s important to have a whole health system perspective, looking at what is happening in the community and in acute settings at the same time.’
Profile:
Alison Holmes is Professor of Infectious Diseases and Director of the NIHR Health Protection Research Unit in Healthcare Associated Infections and AMR, and the Centre for Antimicrobial Optimisation (CAMO) at Imperial College London. She leads an international, multidisciplinary infectious disease research programme focusing on optimising antimicrobial use by developing innovative approaches and technologies to manage and prevent infections.
06.01.2022