Article • Transplants
Cell-free DNA offers several advantages
As part of a national, joint research project in cooperation with Chronix Biomedical (San Jose, CA/USA/Göttingen/Germany), Professor Michael Oellerich MD is on new biomarkers in organ transplantation, aiming to develop personalised immunosuppression for patients. This also entails the development of molecular test procedures, among others for the early detection of rejection. The keyword here is cell-free DNA (cfDNA).
The worldwide problem for transplantation is the lack of donor organs. ‘Therefore,’ Professor Michael Oellerich explains, ‘we have to expand the range of donors in transplant medicine and must also use organs from marginal donors under considerations of the risks involved. The rate of transplant rejection within the first year is up to 20% for liver transplants, 27% for lung transplants and 29% for heart transplants. For the latter, 11% of deaths within the first three years are due to rejection, which therefore poses a significant risk.
‘The survival time of transplants is important for long-term outcome. For the liver and kidney the average is 13 years. The average survival of patients with heart transplants is 11 years. If we could extend the half-life many more people would benefit from a new transplant and the general shortage could be overcome, not to mention the costs. The limiting factors are the irreversible chronic rejection on one hand and, on the other, side effects, such as nephrotoxicity, cardiovascular disease, infections and malignant tumours. Secondary complications are also dangerous and clearly contribute to the difficult prognosis. The problem we continue to deal with is over- and under-immunosuppression. Measuring the cell-free DNA allows us to continuously monitor the integrity of the transplanted organ and so to reduce the number of rejections whilst lowering the side effects through optimisation of immunosuppression,’ Oellerich explains.
The procedure
A method has been developed based on droplet digital PCR, which allows cost-effective monitoring of graft-derived circulating cell-free DNA with same-day turnaround.
Prof. Michael Oellerich
‘If a patient has been under-immunosuppressed after a kidney transplant, for a period of time, for instance, he can develop donor-specific antibodies, which will destroy the organ in the course of time. Drug monitoring allows us to detect the toxicity better, i.e. the over-immunosuppression. However, the procedure is less suitable to assessment of immunosuppression effectiveness, meaning the minimum exposure required cannot be safely predicted. To escape this dilemma we need a parameter that can reveal something about the state of the transplant at any given time. There are no reliable conventional markers for rejection as yet: liver enzymes are not sufficiently specific, there are no suitable non-invasive markers for the heart and lungs and the kidneys can already be affected by a fifty percent loss of function before a rise in creatinine can be detected. In other words, we’d like to improve and personalise immunosuppression through early detection of rejection so that we can also diagnose slow, continuous damage to the transplant at the subclinical stage and prevent chronic loss. We need a test that’s practicable and can be quickly carried out at a reasonable cost.
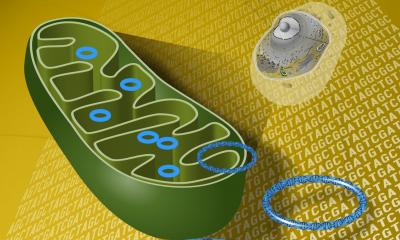
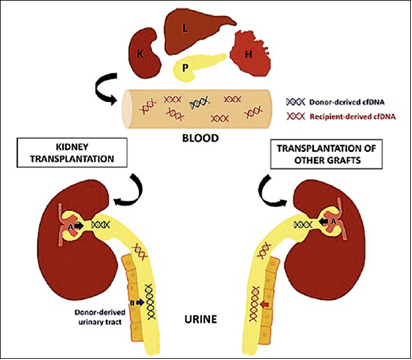
‘Cell-free DNA is extremely suitable for this. The cell nucleus of a damaged cell releases DNA into the blood which can be used as a marker for cell necrosis or apoptosis; it is a marker which indicates the destruction of the tissue. If, for instance, a liver cell dies and the cell nucleus is destroyed, the nucleosomes are flushed into the blood. In the case of necrosis these show up as large fragments and as smaller ones in apoptosis. Against the background that organ transplants are always also genome transplants, i.e. that the donor and recipient genomes are different, there is a fantastic opportunity to detect the genomic DNA of the donor in the recipient’s blood, even though it is present in much smaller quantities. If we detect an increased amount of cell-free donor DNA in the blood we know that the transplanted organ is not doing well.’
How the test works
‘Circulating cell-free DNA in the blood has a very short half-life of four to 30 minutes. In studies, we have examined patients’ stable phases to determine a ratio as to how high the fraction of the circulating donor DNA can be. We then use this as a threshold above which it indicated that the organ becomes damaged. The fraction of donor DNA is very high immediately after a transplantation because of the ischemia/reperfusion damage, but it quickly decreases. If the values rise significantly again over time this is a sign of rejection or other damage to the organ (severe ischemia, for instance). A method has been developed based on droplet digital PCR, which allows cost-effective monitoring of graft-derived circulating cell-free DNA with same-day turnaround. Donor-and recipient DNA are amplified in the droplets and quantified in a flow cytometer via the emission of different fluorescence signals. This procedure has quickly proved itself of value in molecular diagnostics.
‘The particular advantage is that we require no donor material for our test. We use e.g. Streck Cell-Free DNA BCT tubes for the blood test, which keep the sample stable for up to seven days at room temperature through stabilisers. The donor DNA can then be extracted from the sample and the recipient DNA is determined via the white blood cells. Using preselected single nucleotide polymorphisms (SNPs) it is possible to obtain respectively heterologous, homozygous SNPs, i.e. SNPs which are different in the donor and recipient. ‘With the help of droplet digital PCR it is then possible to determine the percentage of cell-free donor DNA.’
Trials held

‘Ten studies have already been published by different centres on the use of graft-derived cell-free DNA in kidney, liver, heart and lung transplantation. We have finished the first multicentre study on liver transplants in 115 patients. The transplant centres at the Hamburg-Eppendorf University Medical Centre, the Charité Berlin and the University Göttingen Medical Centre took part in the study. Most rejections recorded in this study occurred between day 20 and 40 after transplantation, making this period particularly suitable for the early detection of looming rejection through serial determination. Patients with rejection had ten times higher values of cfDNA than stable patients, and an increase in cfDNA was already detectable 8-15 days before rejection. The diagnostic sensitivity of cfDNA was 90% and the specificity was 95%.
‘cfDNA allows a considerably improved identification of acute rejection compared to conventional liver function tests. We have also seen that under-immunosuppression in the first weeks after liver transplantation leads to a significant increase of cfDNA, which can possibly result in negative aspects for long-term survival of the organs. Further comprehensive prospective clinical trials for heart transplants are being carried out with the Bad Oeynhausen Heart and Diabetes Centre NRW and for kidney transplants with the Klinikum Stuttgart.
‘Cell-free DNA offers a number of advantages: It facilitates the detection of subclinical and clinical rejection episodes at an actionable stage, the detection of slow, continuous transplant damage which can lead to chronic dysfunction, the detection of compliance problems with the administration of immunosuppressives and better personalisation of immunosuppression. Based on our experience so far, cfDNA can make a valuable contribution towards shifting the focus from reaction to prevention and saving costs through the avoidance of complications due to improved identification of complications after transplantation. After all, the objective is to improve the outcome based on the maxim ‘value for outcome’ and to use this as guidance for clinical decision making.’
Profile:
Chemical pathologist Michael Oellerich earned a string of credits to his name: MD, HonMD, FACB, FAMM, FFPath (RCPI), FRCPath. Since 2012 he has been a Lower Saxony Distinguished Professor of Clinical Chemistry at the Department of Clinical Pharmacology, Medical Faculty (UMG), at George-August University, Göttingen. At UMG he also chaired the Clinical Chemistry Department/Central Laboratory, and was Dean of the Faculty of Medicine. Presidencies of national and international professional organisations include IATDMCT, DGLM, DGKL, WASPaLM, and from 2003 he has been Editor-in-Chief of Therapeutic Drug Monitoring, since his current research lies in that field, particularly focusing on endogenous biomarkers to achieve personalised immunosuppression in transplantation (e.g. graft-derived circulating cell-free DNA as ‘liquid biopsy’), as well as pharmacogenetics. He has authored over 400 publications and received the Ludolf-Krehl Award, IATDMCT Charles Pippenger Award, WASPaLM Medal of Honour, and WASPaLM Gold-Headed Cane) etc.
25.07.2016