News • Update improves testing
Breast cancer guideline identifies most promising therapies
The updated guideline for estrogen and progesterone receptor (ER/PgR) testing in breast cancer, published jointly by the College of American Pathologists (CAP) and the American Society of Clinical Oncology (ASCO), reaffirms much of the original guidance and has more specific recommendations for handling and reporting cases with low ER expression.
Image source: © Scientific Animations, Inc.
Globally, more than 1 million women are diagnosed annually with breast cancer, and receptor testing conducted on their biopsies typically shows that approximately eight in 10 of these women have ER-positive breast cancer. Well-performed tests are essential to identify those patients who could benefit from endocrine therapy. And more broadly, hormone receptor status can be valuable for tumor classification and other factors that inform treatment.
The update to this pivotal evidence-based guideline is available in an early online release from the Archives of Pathology & Laboratory Medicine and Journal of Clinical Oncology.
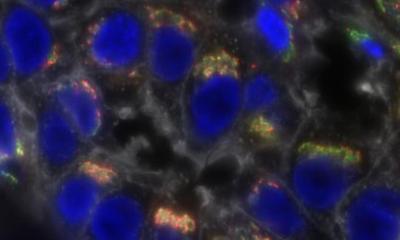
In a notable change, cases with 1-10% of cells staining for ER expression will now be reported as ER-low positive. Pathology reports for these cases should include a recommended comment that acknowledges the more limited data on endocrine responsiveness in this group. Also in such cases, pathologists should report the status of internal controls, with a special comment for specimens that lack internal positive controls.
The new guideline also recommends that laboratories establish specific standard operating procedures to ensure the validity of ER-low positive (1-10%) or ER negative (0 or <1%) results and their interpretation. Correlation of ER staining with the histologic features, and attention to other standard quality control measures, is also recommended, including additional steps to assess unusual or discordant results. The utility of PgR testing continues to be largely prognostic in the ER-positive invasive cancer population, but testing using similar principles to ER testing is still recommended for invasive cancers.
The updated guideline also more clearly recommends that ER be tested in cases of newly diagnosed DCIS (without invasion) to help estimate potential benefit of endocrine therapy to reduce the risk of a future breast cancer event. But, specifically, PgR testing in DCIS is optional. “Like the original guideline, invasive cancers with 1-100% ER staining are considered positive and eligible for endocrine therapy,” Kimberly Allison, MD, FCAP, pathologist and project co-chair explained. “But this update focuses on better assessment of cases with lower levels of ER expression. We wanted to establish guidance to help ensure that the results from hormone receptor testing in women diagnosed with breast cancer are as accurate as possible. This will give physicians and patients more information to guide them when making treatment decisions.”
“The critical point for clinicians is that they need to be aware of and able to discuss with patients the implications of ER-low positive test results,” explained Antonio Wolff, MD, FACP, FASCO, an oncologist representing ASCO and project co-chair. “We now have more standardized recommendations for these cases.” Likewise, patients should be aware of their test results and should be encouraged to discuss decisions to use endocrine therapy with their clinical care team.
Source: College of American Pathologists
15.01.2020