Source: Beckman Coulter
News • Dry Unitized Reagent Assays
Beckman Coulter presents online cost benefit tool at AACC 2019
Beckman Coulter Life Sciences launched the online DURA Innovations calculator tool, a powerful cost benefit analysis resource to accompany its latest Cost Benefit Calculator Handbook.
Beckman Coulter Life Sciences is on booth # 3201 at the 71st American Association for Clinical Chemistry (AACC) Annual Scientific Meeting and Clinical Laboratory Expo (CLE) in Anaheim, California, from August 4 to 8, 2019.
The free online tool enables laboratories to quantify the value of converting to a standardized flow cytometry workflow using the company’s dry reagent technology. The new calculator enables customers using liquid reagents to see for themselves how certain practices actually increase waste and reduce efficiency by affecting workflow. By answering nine key questions, a lab can map out and analyze the cost of its own unique workflow – and then identify the savings to be made by switching to dry reagent technology.
Research indicates that a significant amount of time spent on preparing cocktails could be avoided if a more efficient approach was adopted, especially to sample preparation, ordering antibodies and inventory management. When the cost of antibodies, pipettes, tubes and time on the flow cytometer are taken into account, DURA Innovations dry technology can deliver a saving of at least 30% per sample i,ii. Beckman Coulter Life Sciences’ research shows an average 25% of the reagents used in a lab is wasted due to errors, spillage and internal quality control requirements.
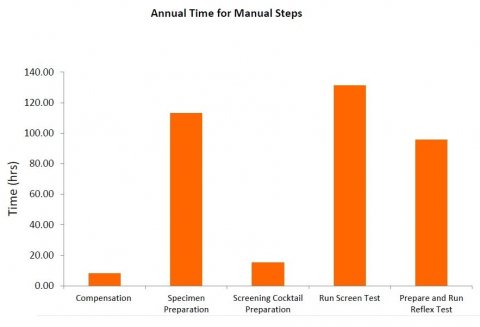
Fig 1: Comparing Time Consuming Manual Steps iii
Source: Beckman Coulter
“Laboratories demand the best possible results with the least available resources. Our business tool enables the flow cytometry lab to demonstrate the financial savings it would make by changing from liquid to dry reagents for its standardized flow analysis,” explained Dr. Mario Koksch, Flow Cytometry Business Unit Vice President and General Manager, Beckman Coulter Life Sciences.
The online calculator tool assumes the use of a multi-color panel that entails cocktail preparation and performing daily assays based on the samples received by a lab per day.
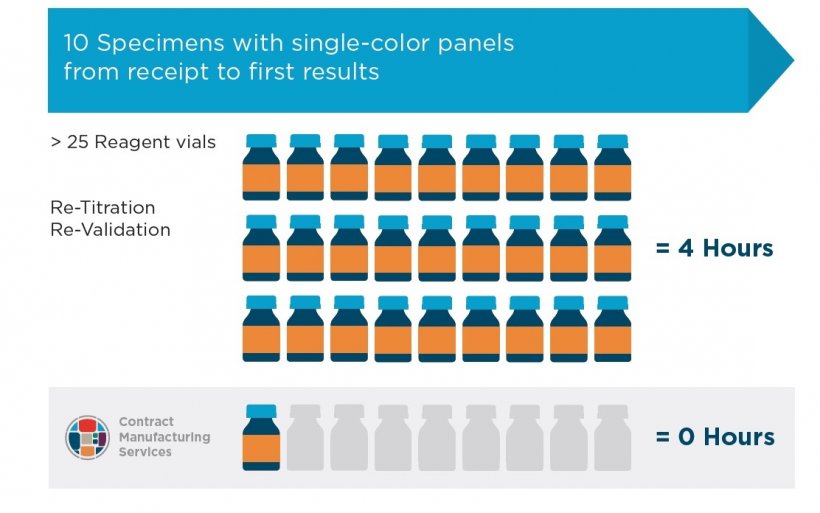
Laboratory staff can spend as much as 90% of their time on routine tasks, such as manually mixing large quantities of cocktails. With single color reagent panels and, say, 10 specimens, it would take approximately four hours from sample receipt to first results. In contrast, dry reagent panels can be delivered ready mixed to the lab’s specific requirement, completely eliminating both labor costs and reagent waste (Fig 1).
The pre-formulated DURA Innovations technology standardizes flow cytometry workflows, helping the lab to simplify sample preparation, by reducing cocktail preparation time and reagent waste. “Having to repeat tests means allocating extra time to mix the cocktail manually – DURA Innovations does this for you. Further, these dry, unitized antibody combinations make it easier to reproduce results and eliminate errors caused by manual antibody pipetting,” added Dr Koksch.
Ready to use panels simplify inventory management removing the need for additional quality checks when single color liquid reagents are used. Also, they help ensure supply expiry issues are avoided, especially when tests have to be quickly repeated. Unlike liquid reagents, DURA Innovations tube kits are stable for 12-18 months when stored at temperatures between 18 and 30°C protected from direct exposure to light, further simplifying transporting and retaining stock levels.
i Rajab A, Axler O, Leung J, Wozniak M, Porwit A. Ten- color 15-antibody flow cytometry panel for immunophenotyping of lymphocyte population. International Journal of Laboratory Hematology 2017 May;39 Suppl 1:76-85. doi: 10.1111/ijlh.12678.
IiBenjamin D. Hedley BD, Keeney M, Popma, J, Chin-Yee I. Novel Lymphocyte Screening Tube Using Dried Monoclonal Antibody Reagents. Cytometry Part B (Clinical Cytometry) 88B:361–370 (2015).
iii Smallwood C1, Galama L1, Apoll L1, Heinrich KH2, Demers J3, Buchanan S3. Poster: Examining the Economic Impact of Laboratory Developed Testing in Flow Cytometry. 2015
Source: Beckman Coulter
06.08.2019