Sponsored • Coronavirus antigen detection
An innovative test for the early diagnosis of Covid-19
Compared to previous SARS and MERS, SARS-CoV-2 became a pandemic due to the high infectivity and different mode of contagion.
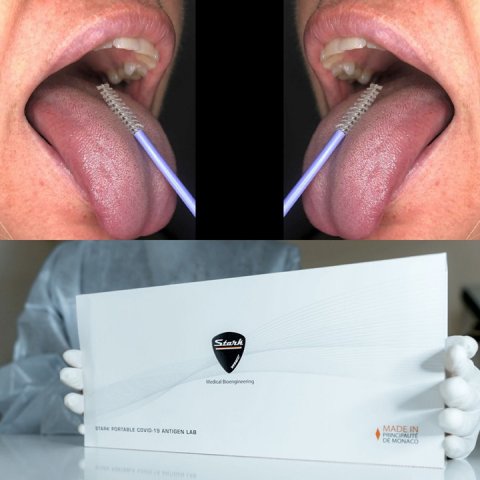
Image source: Stark
SARS-CoV-2 spreads more because it is contagious also before symptoms, during incubation phase. No tests to date have been able to find the virus during the pre-symptomatic stage. Stark has developed a new, rapid, non-invasive method "Stark Portable Covid-19 Antigen Lab”, with a larger diagnostic window able to find Coronavirus antigens also in the pre-symptomatic period.
This is possible using a new method to collect virus antigens and also using a precise and well-proven laboratory method, ELISA test, a gold standard since 1970. The sensitivity reaches LOG-9. This test was made portable by a new patent from Stark (Stark S.a.r.l. Monaco - www.stark.mc). During the initial phase of infection, the SARS-CoV-2 virus infects target cells and replicates within them. In this phase the viral antigens are already present in the cells but not yet in the organic liquids. For this reason this innovative antigenic test does not use a nasopharyngeal swab but a cytological sampling taken in the mouth along with a small amount of saliva, using a non invasive cytobrush able to collect hundred thousands of cells on the tongue. Viral antigens are found in high concentrations in target cells during the pre-symptomatic phase of infection, while the presence of antigens in saliva or mucus increases only later, during the symptomatic phase. Using Stark method with a single test it is possible to analyze two distinct phases of the infection at the same time, widening the diagnostic window and improving precision. The development of the test, is easy and takes place in 30 minutes. A single operator is able to develop up to 40 tests per hour making test management quick and cost-effective.
According to studies, the test is able to detect contagious carriers in pre-symptomatic phase, when protective cellular immunity is capable of defeating 60% of infections before they become symptomatic. This may free many patients from the need for a lengthy quarantine. This test may increase the length of validity of the green certificate for covid-19 by several days, improves the management of emergency room entrances, reduces reception staff, streamlines access and reduces quarantine areas. It improves the management of operators and patients in hospitals and nursing homes and reduces the risk of accepting patients in disease incubation in clean wards. The cyto-salivary swab is non-invasive and is suitable for children and the frail. Simplifies and speed testing procedures for accesses in the countries, for passengers on long travels by finding any subjects in incubation with disease that could become contagious during the journey.
Bibliography
Source: Stark S.a.r.l.
18.11.2021





