News • Improving Alzheimer’s disease imaging
Diagnostic sensors overcome blood-brain barrier
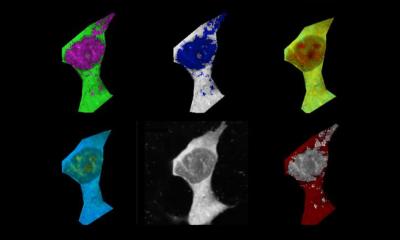
Neurotransmitter levels in the brain can indicate brain health and neurodegenerative diseases like Alzheimer’s.
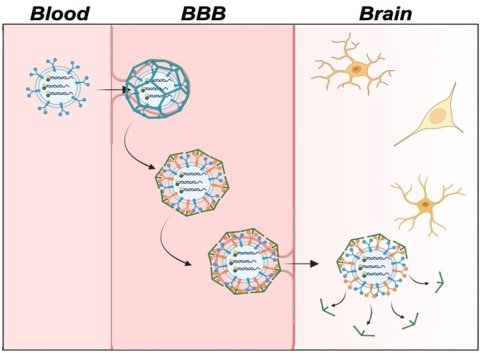
Image credit: Adapted from ACS Central Science 2024, DOI: 10.1021/acscentsci.4c00563
However, the protective blood-brain barrier (BBB) makes delivering fluorescent sensors that can detect these small molecules to the brain difficult. Now, publishing in ACS Central Science, researchers demonstrate a way of packaging these sensors for easy passage across the BBB in mice, allowing for improved brain imaging. With further development, the technology could help advance Alzheimer’s disease diagnosis and treatment.
It is common for neurotransmitter levels to decrease with age, but low levels of the neurotransmitter adenosine triphosphate (ATP) can be an indication of Alzheimer’s disease. To measure the location and amount of ATP in the brain, researchers have developed fluorescent sensors from pieces of DNA called aptamers that light up when they bind to a target molecule. Methods for delivering these sensors from the bloodstream to the brain have been developed, but most contain synthetic components that can’t easily cross the BBB. To develop sensors for live brain imaging, Dr Yi Lu, professor at the University of Texas at Austin's Department of Chemistry, and colleagues encapsulated an ATP aptamer sensor in brain-cell derived microscopic vesicles called exosomes. They tested the new sensor delivery system in lab models of the BBB and in mouse models of Alzheimer’s disease.
Recommended article
Article • Cerebral insights
The brain: mysterious grey matter
More than 80 billion neurons, trillions of synapses and almost 6 kilometres of neural pathways: The brain is an anatomical masterpiece – and still puzzles science. Keep reading to find out about latest research and therapies of brain diseases.
The BBB laboratory model consisted of a layer of endothelial cells on top of a solution containing brain cells. The researchers’ sensor-loaded exosomes were nearly four times more efficient than conventional sensor delivery systems at passing through the endothelial barrier and releasing the fluorescent sensor into the brain cells. This was confirmed by measuring the observed level of ATP-binding-induced fluorescence. Next, Lu’s team injected mouse models of Alzheimer’s disease with either the sensor-loaded exosomes or free-floating unloaded sensors. By measuring fluorescence signals in the mice, the researchers found that the free-floating sensors stayed mainly in the blood, liver, kidneys and lungs, while sensors delivered via exosomes accumulated in the brain.
In mouse models of Alzheimer’s disease, the exosome-delivered sensors identified the location and concentration of ATP in different regions of the brain. Specifically, they observed low levels of ATP in the hippocampus, cortex and subiculum regions of the brain, which are indicative of the disease. The researchers say that their exosome-loaded ATP-reactive sensors show promise for non-invasive live brain imaging and could be developed further to create sensors for a range of clinically relevant neurotransmitters.
Source: American Chemical Society
31.07.2024