News • Rapid diagnostics
A test diagnoses COVID-19 virus in 30 minutes
A new diagnostic test can detect the RNA of SARS-CoV-2, the virus that causes COVID-19, in urine, blood, saliva or mouth swab samples in just 30 to 45 minutes, according to a new study in the open-access journal PLOS ONE by Laura Lamb of the Beaumont Health System, Michigan, USA, and colleagues.
SARS-CoV-2 is difficult to diagnose early in infection as patients may be asymptomatic or have mild, non-specific symptoms. The current standard for diagnostic molecular is quantitative reverse transcription PCR (qRT-PCR), which requires expensive equipment and trained personnel only available in limited laboratories. Reverse transcription loop-mediated isothermal amplification (RT-LAMP) is a one-step technology with fewer barriers to use than qRT-PCR; reagents are inexpensive and can be stored at room temperature, the technique works with a range of sample types, and it has been found to be highly specific, sensitive, and fast for other infectious diseases.
In the new study, researchers developed an RT-LAMP diagnostic test for SARS-CoV-2 and measured the effectiveness of the test on blood, urine, saliva and mouth swab samples that had been spiked with different amounts of SARS-CoV-2 genetic material. The test was also used on clinical samples from COVID-19 patients.
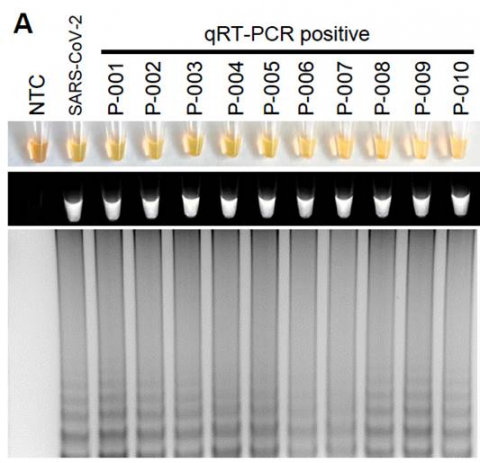
The RT-LAMP test for SARS-CoV-2 successfully detected the virus in all human sample types that were spiked with SARS-CoV-2 genetic material, within 30 to 45 minutes, with an estimated limit of detection of as few as 304 viral copies in a sample. Importantly, the test did not detect virus in samples spiked with RNA other coronaviruses, demonstrating specificity for SARS-CoV-2. In clinical mouth swab samples, RT-LAMP was positive for 95% (19/20) of samples positive by qRT-PCR and negative for 90% (18/20) of samples that were negative by qRT-PCR. The researchers note that those discrepancies between the methods could represent either false positives by the RT-LAMP, contamination or increased sensitivity of RT-LAMP compared to qRT-PCR. The current study was not powered to determine sensitivity in a clinical population.
Lamb summarizes: “This method can rapidly detect the virus for COVID-19 in clinical samples without expensive equipment.”
Source: PLOS
15.06.2020