Image source: Shutterstock/Kateryna_Kon
News • Natural nanocapsules
A new approach for tackling superbugs – without antibiotics
Scientists have uncovered a novel antibiotic-free approach that could help prevent and treat one of the most widespread bacterial pathogens, using nanocapsules made of natural ingredients.
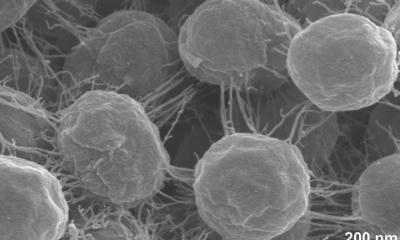
Helicobacter pylori is a bacterial pathogen carried by 4.4 billion people worldwide, with the highest prevalence in Africa, Latin America and the Caribbean. Although the majority of infections show no symptoms, if left untreated the pathogen can cause chronic inflammation of the stomach lining, ulcers and is associated with an increased risk of gastric cancer.
A 'priority pathogen'
Antimicrobial resistance is one of the biggest challenges facing the world and it is predicted to cause more deaths than cancer by the year 2050 unless urgent action is taken
Francisco Goycoolea
In 2017, the World Health Organisation included H. pylori on its list of antibiotic-resistant "priority pathogens" – a catalogue of bacteria that pose the greatest threat to human health and that urgently need new treatments. Current treatments involve multi-target therapy with a combination of antibiotics, but this has promoted the emergence of resistant strains.
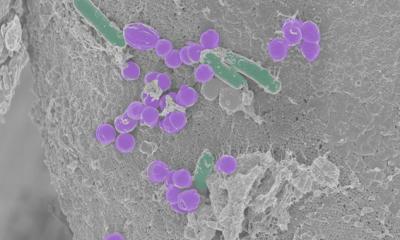
Now, UK and German scientists have uncovered a novel antibiotic-free approach using only food- and pharmaceutical-grade ingredients, which are non-toxic and safe for consumption, to be used as a supplement to complement antibiotic current therapies. “Small capsules made of natural ingredients could offer a new means to deter a globally-spread ‘superbug’ pathogen”, says Professor Francisco Goycoolea from the School of Food Science and Nutrition at the University of Leeds. The formulation is delivered through billions of bundled together nanocapsules, which are smaller than a human blood cell, and prevents the bacteria from attaching to and infecting the stomach cells.
The team, which includes researchers from the universities of Leeds, Münster and Erlangen, hope the nanocapsules could be used as a preventative measure, as well as helping eradicate H. pylori and reduce antibiotic resistant strains. Study co-author Professor Francisco Goycoolea said: “Antimicrobial resistance is one of the biggest challenges facing the world and it is predicted to cause more deaths than cancer by the year 2050 unless urgent action is taken. Helicobacter pylori is a globally-spread pathogen. It is estimated that up to 70% of people host this pathogen worldwide. The bacteria hide under the gastric mucus layer where antibiotics do not penetrate effectively. This often leads to recurrent infections and gives rise to resistant strains. New integral approaches are needed to tackle antimicrobial resistance and research into alternatives to antibiotics is vital. This novel formulation, consisting of small capsules made of natural ingredients, could offer a new means to deter a globally-spread ‘superbug’ pathogen.”
Recommended article
Article • Medical Training
Diagnosing gastrointestinal infections
The human gut literally teems with microorganisms from at least 1,000 different species that are increasingly considered to be a valuable resource for the prediction, aetiology and prognosis of disease. Due to continual contact with the environment, primarily via food, the gut is susceptible to infection when a virus, parasite or bacterium enters and disrupts normal gut microbiota (or flora).
The research, published in the journal ACS Applied Bio Materials, was carried out in vitro – using bacteria and stomach cells outside a human body.
The nanocapsules are loaded with curcumin – a natural compound found in turmeric which has well-documented anti-inflammatory and anti-tumour properties(Article in German). The capsules are coated with lysozyme, an enzyme that helps prevent bacterial infections, and a very low concentration of dextran sulfate, a water-soluble polysaccharide that binds receptors in the bacteria and in the mucosal layer that coats the stomach. The nanopsules are bundled together in the required dose and the formulation prevents the bacteria from adhering to the stomach cells. The team has filed a patent based on this formulation.
Study co-author Professor Andreas Hensel from the Institute for Pharmaceutical Biology and Phytochemistry of University of Münster said: “Standard antibiotics used in today’s clinical practice are quite broad acting compounds, disturbing cell wall architecture, protein formation of membrane integrity. A new generation of antibacterials might be based on more specific molecular targets of the bacteria, acting probably not as broad as the older compounds, but therefore more precisely against specific virulence factors of specific bacteria. The research published in ACS Applied Bio Materials might pinpoint a new way towards controlled drug targeting against H. pylori and its specific adhesion and virulence factors.”
Source: University of Leeds
17.10.2019