News • Repairing heart muscle with stem cells
Trial run for tissue engineered 'heart patch'
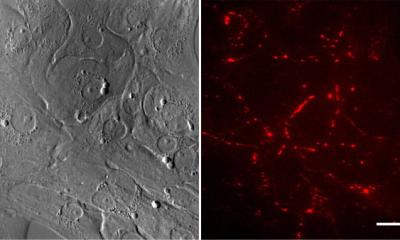
For the first time, engineered heart muscle (EHM) from human induced pluripotent stem cells (iPSCs) will be used to treat patients with heart failure.
After regulatory approval, recruitment of the first patient for the first-in-class, first-in-patient BioVAT-HF early clinical trial has started in Göttingen, Germany. The trial is sponsored by the University Medical Center Göttingen and funded by the German Center for Cardiovascular Research (DZHK) and the Repairon GmbH. Additional participating centers include the University Medical Center Schleswig-Holstein in Lübeck and the Heart- and Diabetes Center of the Ruhr University of Bochum in Bad Oeynhausen.
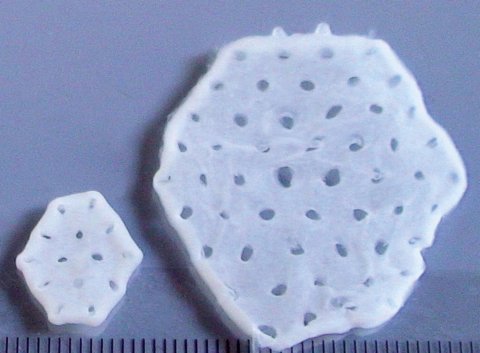
Heart failure affects 60 million people worldwide. With current treatment and medical care, one out of five patients with heart failure will die within 12 months – there is no cure. This means that half of those diagnosed with heart failure today will not be alive in 5 years. The BioVAT-HF-DZHK20** Phase I/II clinical trial comes after 25 years of compre-hensive preclinical development, and is the first to investigate whether the implantation of a heart muscle patch, grown in a laboratory from stem cells, can safely and effectively repair the failing heart.
Approval of the BioVAT-HF-DZHK20 multicenter trial was granted by the Paul-Ehrlich-Institute at the end of 2020. The trial is scheduled to enroll 53 patients with advanced heart failure.
Additional information on the trial can be found on ClinicalTrials.gov (Identifier: NCT04396899)
Source: University Medical Center Göttingen
10.02.2021