Transdermal Carrier Technology (TPM): Non-invasive application of insulin
Phosphagenics' TPM uses natural dermal transport mechanisms to deliver a dose of medication through the skin into the bloodstream. Now a Phase 1b transdermal insulin trial was completed successfully.
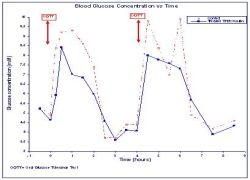
Figure showing Mean Blood Glucose Concentration vs Time.
Phosphagenics Limited develops its TPM/Insulin formulation to enable diabetics the “needle free” application of insulin. 45 volunteers took part in a Phase 1b clinical trial at the Royal Adelaide Hospital in South Australia. Levels of blood glucose, endogenous insulin and C-peptide were measured in the trial, which was executed by the independent clinical research organization CMAX.
“The trial showed that our TPM/Insulin formulation safely penetrated through the human skin and delivered insulin into the bloodstream over a sustained period of time,” said Dr. Esra Ogru, Executive Vice President of Research and Development at Phosphagenics.
The Melbourne-based company prepares a single-blinded, placebo controlled, randomized Phase 2 clinical trial to confirm pharmacodynamics and pharmacokinetics. Associate Professor William Hsu, Joslin Diabetes Centre (Hardvard Medical School), and Dr. Sepehr Shakib, Director at the Department of Clinical Pharmacology at the Royal Adelaide Hospital, will guide the Phase 2 study. Final results are expected by the end of the first quarter, 2008.
Dr. Ogru: “We believe that these results are indicative of Phosphagenics’ potential to provide the millions of insulin-dependent diabetics with a non-intrusive alternative to multiple needle injections each day.”
For more informations, please visit Phosphagenic’s website at http://www.phosphagenics.com
09.08.2007