The PARTNER Trial
Cohort A and B Results. Aortic stenosis is characterised by the hardening and narrowing of the aortic valve that pumps blood into the body’s main artery. It affects nearly 5% of those over 75 in Europe, with an estimated 16,000 Britons suffering from severe aortic stenosis.
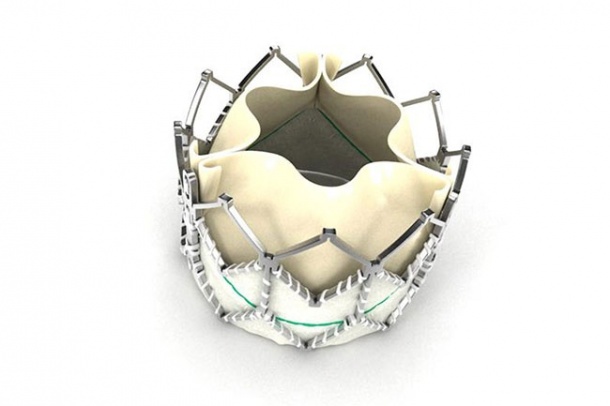

For those with severe disease, without surgical intervention, the prognosis is extremely poor and the three-year survival rates are less than 30%. In Europe, transcatheter aortic valve implantation (TAVI) is now an established, evidence-based, alternative to open aortic valve replacement in patients with aortic stenosis who are unsuitable for conventional cardiac surgery. Over 1,500 procedures have now been performed in the UK with outcomes that match or exceed international norms.
The PARTNER trial
The Placement of AoRTic traNscathetER valves (PARTNER) trial evaluated the Edwards Sapien valve in patients with aortic stenosis who are considered either high-risk or inoperable for conventional open-heart valve surgery. Within the trial, Cohort B included 358 patients with severe, symptomatic aortic stenosis deemed inoperable for traditional open-heart surgery. Patients were evenly randomised to receive either the Edwards Sapien valve or standard therapy. Cohort A involved 699 patients with severe, symptomatic aortic stenosis deemed at high risk for traditional open-heart surgery. Patients were evenly randomised to receive either the Edwards Sapien valve with transfemoral or transapical delivery or traditional open heart surgery.
Cohort B
These data were previously released in September 2010 and published in the New England Journal of Medicine and found that rate of death from any cause at one year was 20% lower with TAVI than with standard therapy. The study’s authors concluded that balloon-expandable TAVI should be the new standard of care for patients with aortic stenosis who are not suitable candidates for surgery.
In November 2010, a quality of life analysis of the PARTNER Cohort B data revealed that patients experienced both cardiovascular and physical health benefits thanks to TAVI. The study’s authors concluded that the physical improvements were roughly comparable to a 10-year reduction in age. On a scale from 0 to 100, where a 20-point improvement is considered substantial, the Edwards transcatheter valve patients had a 25-point improvement in quality of life scores compared to the control group at one year.
Cohort A
The results of a pivotal clinical study of high-risk surgical patients with severe aortic stenosis treated in Cohort A of the trial were announced at the 2011 American College of Cardiology’s (ACC) 60th Annual Scientific Session & Expo in New Orleans, US A. The data demonstrated that the study achieved its primary endpoint at one year, concluding that survival of patients treated with the Edwards Sapien transcatheter aortic valve
was equivalent to those treated with surgical aortic valve replacement.
In this cohort, the study found that TAVI was non-inferior to surgical aortic valve replacement (AVR) for all-cause mortality at one year, 24.2% v. 26.8 % respectively. In addition, mortality at 30 days was lower than expected in both arms of the trial, with TAVI at 3.4% and AVR at 6.5%. The observed mortality in these AVR patients was lower than the predicted risk of operative mortality of 11.8%.
Overall, the trial data has shown that TAVI gives patients substantially better quality of life whilst achieving a survival rate at one year equivalent to that of conventional surgery and significantly better than for patients who receive standard care.
####
Chohort A results were also mentioned in The new England Journal of Medicine. 9. June 2011 - vol. 364 no. 23
30.04.2012