Healing hearts 2
Stem cell cocktails
Nothing new has been invented in heart failure in the last 15 years, according to Christian Homsy, CEO of Belgian-based Cardio3 Biosciences. This explains the excitement surrounding an emerging treatment among cardiologists, patients and investors.

The innovative technique for turning stem cells into cardiac muscle was developed at the celebrated Mayo Clinic in the United States.
One of the first cardiologists to get caught up in the excitement over this new approach was William Wijns MD, from the Cardiovascular Centre Aalst in Belgium. In 2007, Wijns and Homsy founded Cardio3 BioSciences in order to license the technology and bring this American innovation to patients suffering chronic heart failure. ‘Dr Wijns is totally out of his element when it comes to questions about the market potential of this new procedure,’ Christian Homsy explained. ‘He’s 120% dedicated to treating patients and the expertise he brings is knowing the outcome needed for patients and how to test it with patients.’
However, investors in Life Sciences businesses, which are very much focused on market potential, also caught the excitement. Homsy was able to gather €60 million in several financing rounds to bring the science out of the laboratory and into the long process of experiments and clinical trials.
Following a successful Phase II study with 45 patients, the company boldly went public in July 2013, raising a further €23 million on the NYSE Euronext stock exchanges in Brussels and Paris. Homsy said this fresh funding will allow Cardio3 to complete its European Phase III study, the final step in an intensive clinical development programme before seeking the regulatory approval that will finally make the treatment available to patients.
The treatment is only available in Europe. With successful results in the Phase III clinical trial, Cardio3 will be able to discuss with the USA’s Food & Drug Administration (FDA) the start of a clinical trial there. Millions of people are waiting, some who may not live long enough to see this therapy arrive in the hospital. In Europe alone, 3.6 million people are diagnosed each year with HF, a very serious condition in which a damaged heart cannot pump enough blood to meet the body’s need. This number is expected to double over the next 10 years. One patient in three who is diagnosed with HF will die within the year.
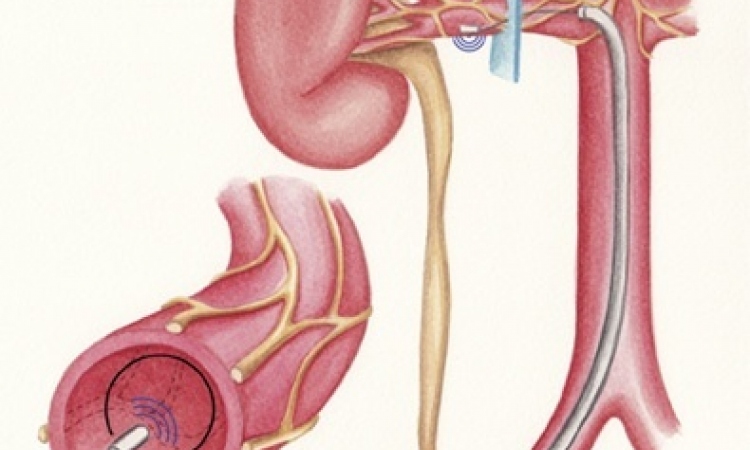
Called C-Cure, the new therapy is a three-step process. First, cells are harvested from the patient’s bone marrow in the hip, using a catheter and performed using local anaesthesia. These cells are sent to Cardio3’s processing centre where they are re-engineered with the ‘cocktail’ invented at the Mayo Clinic. Called Cardiopoiesis, the reprogramming process takes 30 days, teaching the new cardiac progenitor cells to behave like those cells that have been lost to heart disease. This highly personalised batch of cells is frozen and sent to the hospital where it can be injected through a minimally invasive procedure into the patient’s heart muscle using a specialised catheter developed by Cardio3.
‘We believe C-Cure has the potential to go beyond symptom relief towards healing heart tissue and could mark a significant step forward in treatment for heart failure patients, Homsy says in the cautious language required before the results of the Phase III trial are known.
In June 2013, the first patients were enrolled in the CHART-1 trial (Congestive Heart failure Cardiopoietic Regenerative Therapy). This is a prospective, multi-centre, randomised, blinded study, comparing treatment with C-Cure to a sham treatment. More than 240 patients with chronic advanced HF of ischemic origin will be enrolled. The primary endpoint of the trial is a composite result at nine months after treatment that includes mortality, morbidity, the Six Minute Walk Test, quality of life, and left ventricular structure and function.
The result from the Phase II trial that generated the excitement behind C-Cure, said Homsy, is that the heart became smaller for treated patients. In chronic HF, the heart progressively becomes larger to keep up with the body’s needs until finally it fails. Among patients in the Phase II trial, ‘it was as if the heart had regressed back to an earlier stage in the disease history, at a moment when the patients were less sick than they are now,’ he explained. In terms of heart function, the heart’s ability to pump blood, measured as the left ventricular ejection fraction, improved by 25% after six months. The improvement in a patient’s physical capability was an increase of 77 metres during the Six Minute Walk Test.‘This progress has not been seen before in a chronic disease,’ said Homsy. ‘We have results out to two years now and this can be considered long-term results for patients who had a 12-month life expectancy. It is not a guarantee of success for the CHART-1 trial, but it points in the right direction.’
‘What is important here, what makes this unique are these results combined with the physical remodelling of the heart,’ he said.
The name of the product, C-CURE derives from the full description of Cardiopoietic stem Cell therapy in heart failURE and, despite its hopeful sound, Homsy cautioned that the therapy it is not a cure. ‘Cure means you become healthy again, that your heart has been reconstructed in its entirety,’ he said. Small animal trials showed that C-Cure could do that, but bringing the treatment into humans is a completely different case. ‘Heart failure in humans is a complex disease,’ he said. ‘We have shown that C-Cure can regress the disease by remodelling the heart. But to heal a patient completely? We are not there yet.’
29.08.2013