Bacteria
Revolutionary antibiotics will save the world
An international team of including the Lomonosov Moscow State University researchers discovered which enzyme enables Escherichia coli bacterium (E. coli) to breathe.
Scientists discovered how the E. coli bacterium can survive in the human gut - earlier the question how they breathe was a mystery to experts. Vitaliy Borisov, Senior Researcher, Doctor of Biological Sciences, Professor of the Russian Academy of Sciences, A.N. Belozersky Research Institute physical and chemical biology employee, the Lomonosov Moscow State University and one of the authors, explains that breathing E. coli uses special enzymes, which are absent in the human body. This means that the discovery of the scientists can contribute to the creation of new drugs, which will be detrimental to the bacteria without harming a human.
The energy for the vital activity of any organism comes from food, and is generated by the means of redox processes in the body. The food is converted into energy not directly but through intermediaries. First, the complex molecules are decomposed into simpler: proteins are decomposed into amino acids, fats - to fatty acids, carbohydrates - to monosaccharides. Oxidation of simpler molecules releases energy, which all is contained in the electrons.
The electrons passes to the respiratory chain with the so-called reducing equivalents (electron-carrying compound). They are NADH (nicotinamide adenine dinucleotide) and ubiquinol, also known as coenzyme Q. These two basic reducing equivalents fully cope with the processing of food: NADH is a water-soluble compound and ubiquinol is fat-soluble. Membranous enzymes accept electrons from reducing equivalents and transfer them to molecular oxygen.
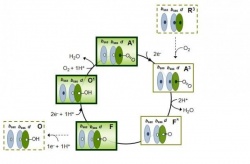
The terminal cytochrome oxidase is the main membrane enzyme responsible for the human mitochondrial respiration and was thought to be used for the breath of E. coli as well. The scheme of oxidases action is simple: transferring electrons to molecular oxygen, reducing equivalents are oxidized again, and as a result "the energy currency" of the cell - the proton-moving force is generated.
'If you stop breathing, you die just because oxygen does not flow to the oxidase, and it does not produce energy,' says Vitaly Borisov.
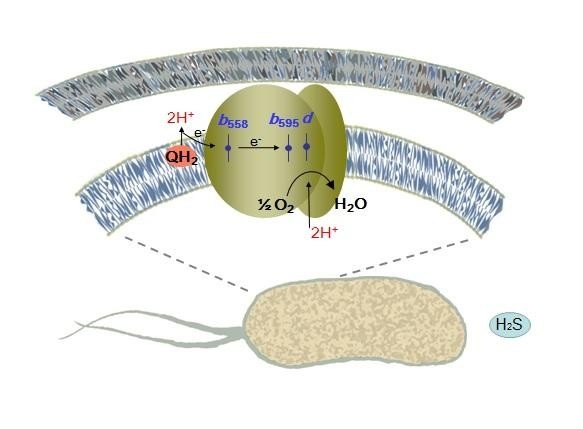
The Escherichia coli bacterium lives in the gastrointestinal tract, where a lot of hydrogen sulfide is produced, which attenuates mitochondrial respiration. Free hydrogen sulfide inhibits cytochrome oxidase work. Its concentration exceeds several hundred times the minimum concentration required for substantial blocking of this enzyme. Hence, it seems that the E. coli bacterium cannot "breathe", but despite that the bacteria somehow survive in the intestine. The researchers assumed that the breath in the presence of hydrogen sulfide is still possible, but due to other oxidase. The fact is that the breath in people and bacteria occur in different ways. Each cell in our body "breathes" due to the work of only the cytochrome-c oxidase, others we have not. However, the E. coli bacteria has two types of oxidase: bo-type cytochrome oxidase (analogue of "human" cytochrome-c oxidase) and completely different bd-type cytochromes.
'Our hypothesis was that the bd-type oxidase (bd-I and bd-II) are more resistant to the hydrogen sulfide inhibition than the bo-type cytochrome oxidase,' commented Vitaly Borisov.
To test this hypothesis scientists needed to learn how the sulfide presence in the environment affects the growth of the E. coli bacteria cells, which have only one terminal oxidase (bd-I, bd-II or bo) in the respiratory chain. a variety of biochemical, biophysical and microbiological methods and approaches were applied, as well as the method of the intended mutagenesis.
The hypothesis was fully confirmed.
'Bo-oxidase's activity is completely inhibited by the hydrogen sulfide, while the work of the bd-oxidases remains untouched by the H2S. Thus, in order to successfully produce the main types of "the energy currency" under a high concentration of hydrogen sulfide, the intestinal microflora inhabitants should use a unique type of terminal oxidases, which is missing in the cells of humans and animals,' says Vitaly Borisov.
The discovery could be used in the future to develop medicines that regulate the intestinal microflora and relieving it from harmful bacteria. As human cells do not contain the bd-type oxidase, the question of the ability to combat disease-causing bacteria without causing harm to the human body becomes relevant. For example, the bacterium causing tuberculosis, which's primarily membrane enzyme is also a bd-type oxidase, quickly gaining resistance to classical antibiotics. Through this study there is a prospect of a new type of antibiotics "turning off" the oxygen only to the harmful bacteria cells, not to human cells.
Source: Lomonosov Moscow State University
10.05.2016