Revised ACC/AHA/ESC guidelines on atrial fibrillation
Stroke risk should determine anti-clotting treatment for people with irregular heartbeat.
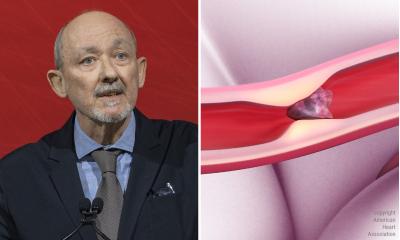
Risk factors for stroke should be used to determine whether anti-clotting therapy is given to people with atrial fibrillation (AF), according to revised Guidelines for the Management of Patients with Atrial Fibrillation released by the American College of Cardiology, American Heart Association and the European Society of Cardiology. (Authors: Amy Murphy at ACC, Bridgette McNeill at AHA, and Lisa Abdolian at ESC).
Atrial fibrillation (AF), the most common heart rhythm disturbance, increases the risk for stroke, heart failure and all causes of death, especially in women. Presently AF affects over 4.5 million Europeans, a number expected to increase even more due to aging populations, a rising number of people with chronic heart disease and improved diagnostic possibilities.
Previous guidelines published in 2001 recommended using several patient characteristics - age, gender, heart disease risk and concurrent conditions - to decide proper anti-clotting therapy for these patients. The new approach recommends that the risk for stroke should be the main factor, said Valentin Fuster MD PhD, co-chair of the guidelines writing committee, fellow of all three associations, and professor of medicine and director of the Mount Sinai Cardiovascular Institute in New York. ‘We focused on stroke risk because AF is associated with increased long-term risk for stroke,’ he said. ‘About 15-20% of strokes occur in people with AF, and those strokes are especially large and disabling. Incorporating existing recommendations on anti-clotting therapy from the stroke primary prevention guidelines will streamline patient care and make recommendations clearer for physicians.’
During the last two decades, hospital admissions in the USA and Europe increased by 66%. Total costs approach ¤13.5 billion in the European Union.
The revised guidelines also recommend daily aspirin therapy (81-325 mg) to guard against blood clots in AF patients with no stroke risk factors. Aspirin or warfarin is recommended for those with one ‘moderate’ risk factor (over age 75, high blood pressure, heart failure, impaired left ventricular systolic function or diabetes). Warfarin is recommended for people with any ‘high’ risk factor (previous stroke, transient ischaemic attack [TIA], systematic embolism or prosthetic heart valve) or more than one moderate risk factor.
According to co-chair Lars E Rydén MD PhD, also a fellow of all associations and professor emeritus at Karolinska Institute, in Stockholm, the guidelines help physicians to prioritise the objectives of patient care according to the following steps: 1) controlling heart rate, 2) preventing clots, and, if possible, 3) correcting the rhythm disturbance. Rate control usually involves achieving a ventricular rate (pulse) of 60 to 80 beats per minute at rest and between 90 and 115 beats per minute during moderate exercise.
Also new in the guidelines, catheter ablation - the procedure to correct irregular heartbeat with radiofrequency energy - is considered ‘a reasonable alternative to drug therapy to treat AF in patients with little or no left atrial enlargement, and in whom drug treatments did not stop the rhythm disturbance,’ Dr Fuster said.
Depending on symptoms, controlling the heart rate may be the reasonable therapy in elderly patients with persistent AF who have hypertension or heart disease, according to the authors. For people under age 70, especially those with recurrent AF and no evidence of underlying heart disease, rhythm control may be the preferred approach, starting with drugs and by means of catheter ablation if medication fails to stop the attacks. Drs Fuster and Rydén emphasised: ‘Regardless of the approach, the need for anti-clotting therapy should still be based on stroke risk and not on whether proper heart rhythm is maintained.’
Other writing committee members: David S Cannon MD; G Neal Kay MD; Harry J Crijns MD; James E Lowe MD; Anne B Curtis MD; S Bertil Olsson MD PhD; Kenneth A Ellenbogen MD; Eric N Prystowsky MD; Jonathan L Halperin MD; Juan Luis Tamargo MD; Jean-Yves Le Heuzey MD; and Samuel Wann MD.
The European Heart Rhythm Association and the Heart Rhythm Society collaborated on the statement.
Full guidelines: http://www.escardio.org/knowledge/guidelines/Management_of_Atrial_Fibrillation.htm and The European Heart Journal
ESC guidance on cardiac rhythm management products
Reporting on the performance and adverse events of Cardiac Rhythm Management (CRM) device technology is significantly different in European Union and non-EU countries. This, according to results from a policy conference held by the ESC’s European Heart Rhythm Association (EHRA), could cause problems for the general public if steps are not taken to minimize them.
Variations in regulatory requirements and approval processes mean that a new CRM product is frequently clinically tested and commercialised much earlier in Europe than elsewhere. The EHRA document states that active monitoring of these products in Europe is therefore necessary and should be conducted independently from international monitoring or registry activities, although data sharing should be encouraged.
National Competent Authorities should be encouraged to work with clinician/scientific societies to improve event reporting on national levels. Specifically, EHRA recommends the creation of a single, standardised multi-lingual incident notification sheet that can standardise the process of reporting incidents or near-incidents. Additionally, a confidential forum for informal discussions of product performance issue should be established to help improve communication about devices.
EHRA also proposed a novel approach for assessing individual hazard analysis. This risk model takes into account the condition of an arrhythmic patient and the current indication for the device implantation, i.e. primary or secondary prevention of sudden cardiac death (for patients with implantable cardioverter-defibrillators). This approach will help prioritise patients who should be contacted in case of a field safety corrective action, as well as provide the best advice to patients.
Finally, EHRA envisions a role for itself in explaining scientific and medical issues associated with such advisory communications to the media and other interested persons.
These observations and ideas outline a framework for future actions on the regulatory and the public policy front and a basis for formulating clinical guidelines. Full text: EUROPACE Journal (5/06).
30.08.2006