Discovery
Researchers reveal cellular clockwork underlying inflammation
Researchers at the Virginia Bioinformatics Institute at Virginia Tech have uncovered key cellular functions that help regulate inflammation -- a discovery that could have important implications for the treatment of allergies, heart disease, and certain forms of cancer.
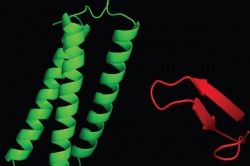
The discovery, to be published in the Oct. 6 issue of the journal Structure, explains how two particular proteins, Tollip and Tom1, work together to contribute to the turnover of cell-surface receptor proteins that trigger inflammation.
"The inflammatory response can be a double-edged sword," said Daniel Capelluto, an associate professor of biological sciences in the College of Science, a Fralin Life Science Institute affiliate, and Fellow in the Virginia Bioinformatics Institute. "At appropriate levels, the inflammatory response protects your body from foreign materials, but if it is not properly regulated it can lead to severe, chronic conditions."
Inflammation plays a role in major health problems such as heart disease, diabetes, Alzheimer's disease, and cancer, as well as psychiatric diseases such as depression and autism spectrum disorder, according to the National Institutes of Health. The discovery by Virginia Bioinformatics Institute researchers reveals protein structural changes within cells that could help inform treatments.
When the body is attacked by a virus or other germs, specialized cells secrete pro-inflammatory signals recognized by interleukin-1 receptors on the surface of specialized cells, triggering the release of additional pro-inflammatory molecules which amplify the response against pathogens. These receptors continue to promote inflammation as long as they detect that initial "alert" signal.
When a healthy level of inflammation is reached, the receptor proteins need to be removed and delivered to intracellular compartments called endosomes for containment and further clearance. "We knew that Tollip and Tom1 worked together on the surface of the endosome to transport these receptors for degradation," said Capelluto. "But it wasn't clear how they fit together structurally or why two separate proteins were needed to fulfill this cargo transport function."
Using a combination of techniques, including two- and three-dimensional nuclear magnetic resonance spectroscopy, surface plasmon resonance, and fluorescence cell microscopy, Capelluto's team determined that Tollip's association with Tom1 drastically changes Tollip's structure, forming a unit that can potentially transport cargo much more efficiently than either protein on its own.
Tollip contains a functional module called C2, which anchors the protein to the surface of the endosomal membrane to pick up cargo. However, with C2 engaged in holding Tollip's position, the protein's load-bearing capacity is limited. But when Tom1 binds to Tollip, Tollip's C2 domain is no longer directly associated with the endosomal membrane, potentially shifting its function from "landing gear" to "cargo bay." The resulting unit can carry larger loads and assist in the clearance of unneeded receptor proteins more efficiently.
The discovery expands fundamental understanding of how the body regulates its inflammatory response and may inform efforts to treat diseases associated with chronic inflammation such as heart disease, stroke, and colon cancer, the researchers said.
Source: Virginia Tech
10.09.2015