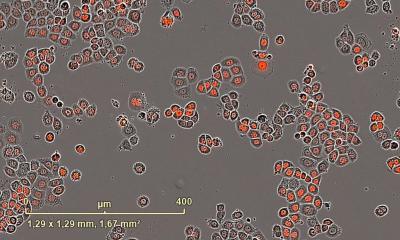
Image source: Roschewski et al., Science Immunology 2020 (CC BY 4.0)
News • BTK inhibitor vs. respiratory distress
Off-label cancer drug shows promise against severe COVID-19
Early data from a clinical study suggest that blocking the Bruton tyrosine kinase (BTK) protein provided clinical benefit to a small group of patients with severe COVID-19.
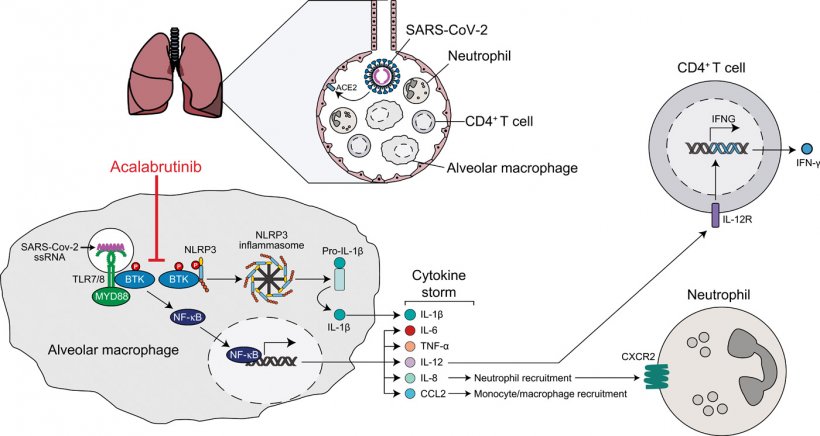
Researchers observed that the off-label use of the cancer drug acalabrutinib, a BTK inhibitor that is approved to treat several blood cancers, was associated with reduced respiratory distress and a reduction in the overactive immune response in most of the treated patients.
The findings were published in Science Immunology. The study was led by researchers in the Center for Cancer Research at the National Cancer Institute (NCI), in collaboration with researchers from the National Institute of Allergy and Infectious Diseases (NIAID), both part of the National Institutes of Health (NIH), as well as the U.S. Department of Defense’s Walter Reed National Military Medical Center, and four other hospitals nationally.
These findings should not be considered clinical advice but are being shared to assist the public health response to COVID-19. While BTK inhibitors are approved to treat certain cancers, they are not approved as a treatment for COVID-19. This strategy must be tested in a randomized, controlled clinical trial in order to understand the best and safest treatment options for patients with severe COVID-19.
The BTK protein plays an important role in the normal immune system, including in macrophages, a type of innate immune cell that can cause inflammation by producing proteins known as cytokines. Cytokines act as chemical messengers that help to stimulate and direct the immune response. In some patients with severe COVID-19, a large amount of cytokines are released in the body all at once, causing the immune system to damage the function of organs such as the lungs, in addition to attacking the infection. This dangerous hyperinflammatory state is known as a “cytokine storm.” At present, there are no proven treatment strategies for this phase of the illness. The study was developed to test whether blocking the BTK protein with acalabrutinib would reduce inflammation and improve the clinical outcome for hospitalized patients with severe COVID-19.
Recommended article
News • Deadly mechanism uncovered
Inside COVID-19's 'cytokine storm'
Leading immunologists in Japan are proposing a possible molecular mechanism that causes massive release of proinflammatory cytokines, or a cytokine storm, leading to the acute respiratory distress syndrome (ARDS) in COVID-19 patients. Their suggestions, published in the journal Immunity, are based on recent findings that explain how SARS-CoV-2 enters human cells.
This prospective off-label clinical study included 19 patients with a confirmed COVID-19 diagnosis that required hospitalization, as well as with low blood-oxygen levels and evidence of inflammation. Of these patients, 11 had been receiving supplemental oxygen for a median of two days, and eight others had been on ventilators for a median of 1.5 (range 1-22) days.
Image source: Scientific Animations (CC BY-SA 4.0)
Within one to three days after they began receiving acalabrutinib, the majority of patients in the supplemental oxygen group experienced a substantial drop in inflammation, and their breathing improved. Eight of these 11 patients were able to come off supplemental oxygen and were discharged from the hospital. Although the benefit of acalabrutinib was less dramatic in patients on ventilators, four of the eight patients were able to come off the ventilator, two of whom were eventually discharged. The authors note that the ventilator patient group was extremely clinically diverse and included patients who had been on a ventilator for prolonged periods of time and had major organ dysfunction. Two of the patients in this group died.
Blood samples from patients in the study showed that levels of interleukin-6 (IL-6), a major cytokine associated with hyperinflammation in severe COVID-19, decreased after treatment with acalabrutinib. Counts of lymphocytes, a type of white blood cell, also rapidly improved in most patients. A low lymphocyte count has been associated with worse outcome for patients with severe COVID-19. The researchers also tested blood cells from patients with severe COVID-19 who were not in the study. In comparison with samples from healthy volunteers, they found that these patients with severe COVID-19 had higher activity of the BTK protein and greater production of IL-6. These findings suggest that acalabrutinib may have been effective because its target, BTK, is hyperactive in severe COVID-19 immune cells.
The results of this study were used to inform the trial design of the CALAVI (acalabrutinib) randomized, controlled clinical trial, sponsored by AstraZeneca, which will examine the safety and efficacy of acalabrutinib in patients with severe COVID-19.
Source: National Cancer Institute (NCI)
08.06.2020