Novel DNA repair mechanism brings new horizons
A group of researchers, lead by Vasily M. Studitsky, professor at the Lomonosov Moscow State University, discovered a new mechanism of DNA repair, which opens up new perspectives for the treatment and prevention of neurodegenerative diseases. The article describing their discovery is published in AAAS' first open access online-only journal Science Advances.
The DNA molecule is chemically unstable giving rise to DNA lesions of different nature. That is why DNA damage detection, signaling and repair, collectively known as the DNA damage response, are needed.
"In higher organisms DNA is bound with proteins in complexes called the nucleosome. Every ~200 base pairs are organized in nucleosomes, consisting of eight histone proteins, which, like the thread on the bobbin, wound double helix of DNA, which is coiled into two supercoiled loops. Part of the surface of the DNA helix is hidden, because it interacts with histones. Our entire genome is packed this way, except for the areas, from which the information is being currently read", says Vasily M. Studitsky , who is the leading researcher and the head of the Laboratory of Regulation of Transcription and Replication at the Biological Faculty of the Lomonosov Moscow State University.
The dense packing allows DNA molecule with a length of about two meters to fit into a microscopic cell nucleus, but it makes significant surfaces of the DNA inaccessible for the repair enzymes -- the proteins that manage the "repair" of damaged DNA regions. The damage of the DNA, if not repaired, leads to accumulation of mutations, cell death, and to the development of various diseases, including neurodegenerative, e.g. Alzheimer's disease.
A group of researchers studied the mechanism of detection of single-stranded DNA breaks at which the connection is lost between nucleotides on one strand in the places where the DNA is associated with histones.
Scientists know quite a lot about the mechanism of the repair. It is known that for the synthesis of a protein, information written in the genetic code, which could be imagined as the manual for its assembly where triples of nucleotides match certain amino acids, should be taken out of the nucleus into the cytoplasm of the cell.
Thin and long strand of the DNA is packed in the nucleus and can tear at the exit to the outside. Moreover, it cannot be sacrificed as the cell's nuclear DNA is is only present in two copies. Therefore, when it is necessary to synthesize specific protein, small region of DNA is unwound, the two strands are disconnected, and the information on the protein structure with one of the DNA strands is written in form of RNA, single-stranded molecule. The mRNA molecule, which serves as the template for making a protein, is synthesized by the principle of complementarity: each nucleotide pair corresponds to another one.
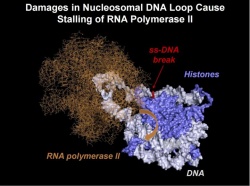
During the transcription of information (its rewriting into RNA) the RNA polymerase enzyme "rides" on the DNA chain, and stops when it finds the break. Like a proofreader of a text, RNA polymerase after it is stalled, triggers a cascade of reactions, resulting in the repair enzymes fixing the damaged area. At the same time, the RNA polymerase cannot detect discontinuities present in the other DNA strand.
"We have shown, not yet in the cell, but in vitro, that the repair of breaks in the other DNA chain, which is "hidden" in the nucleosome, is still possible. According to our hypothesis, it occurs due to the formation of special small DNA loops in the nucleosome, although normally DNA wounds around the histone "spool" very tightly", says Studitsky. "The loops form when the DNA is coiled back on nucleosome together with polymerase. RNA polymerase can "crawl" along the DNA loops nearly as well as on histone-free DNA regions, but when it stops near locations of the DNA breaks, it "panics", triggering the cascade of reactions to start DNA "repairs".
During the experiment, special sites, where single-stranded breaks can be introduced by adding specific enzymes in a test tube, were inserted into the DNA. Then a single nucleosome transcribed by a single RNA molecule was studied. In this model system, which was developed in 2002 by the same group of scientists, histones were assembled on the molecule with an accuracy within one nucleotide. Having specially introduced breaks at precise locations on the DNA, the researchers examined the impact of breaks on the progression of the RNA polymerase. It turned out that only in nucleosomes, rather than in the histone-free DNA, the enzyme stopped, when the break was present in the other DNA strand. Wherein it did not stop before the break, but immediately after it. It was difficult enough to understand the mechanism that allows it to notice the damage at the "back" of RNA polymerase, as if it had "eyes on the back of the head", although, obviously, it does not have neither one nor the other.
The analysis of breaks in different positions allowed to hypothesize that stalling of RNA polymerase is caused by the formation of the loop, which blocks movement of the enzyme. The findings open up a new direction for the work on the subject of DNA repair.
Previously the role of chromatin considered passive as scientists thought the DNA repair is possible only on histone-free DNA. However, Studitsky and his colleagues found that the thread can be repaired without complete unwinding of DNA "coils". The highly conserved histones play an important role in this process as changes in their structure are rejected by natural selection. Moreover, the high level of protein conservation just assumes its active participation in many processes.
Furthermore, the models proposed by the scientists first time ever explains the role of the so-called topological locks, which are formed during the passage of any enzyme along the DNA when it meets a nucleosome.
"In terms of applied science discovery of a new mechanism of reparation promises new prospective methods of prevention and treatment of diseases. We have shown that the formation of loops, which stop the polymerase, depends on its contacts with histones. If you make them more robust, it will increase the efficiency of the formation of loops and the probability of repair, which in turn will reduce the risk of disease. If these contacts are destabilized, then by using special methods of drug delivery you can program the death of the affected cells", Studitsky concluded, adding that the process of development and testing of such drugs, of course, requires considerable time.
Source: Lomonosov Moscow State University
06.07.2015